
Thermal lasers have achieved extraordinary results in microprecision manufacturing of medical implants and devices the past 20 years. Devices we take for granted today, such as vascular stents, could not be produced without the technology; however, there are still significant limitations on what medical implant and device manufactures can produce using thermal lasers.
One major issue inherent in thermal laser machining is heat, because heat:
• Introduces more variability in the manufacturing output
• Requires additional material to produce a finished product
• Damages the material and increase the potential for failure related to metal fatigue in high stress regions
• Requires post-production finishing that can add unnecessary time and cost
The solution to this issue is to remove the heat from the manufacturing process. Athermal laser machining, the next generation technology advancement in medical and implant manufacturing, delivers this solution.
Athermal lasers address the limitations of thermal laser technology in all metals, including Nitinol. Additionally, athermal laser technology enables the machining of bioresorbable materials, which are interchangeably referred to as bioabsorbable, biodegradable and resorbable. Since these materials are absorbed into the bloodstream over time, this family of bioresorbable materials represent the next major advancement for orthopedic applications where the implant is needed only temporarily, such as with vascular scaffolds.
Evolving medical implant design has moved beyond historical manufacturing methods, demanding new, highly advanced manufacturing technologies. Athermal laser machining is a critical manufacturing advancement that enables the machining of these evolving implants and devices that would be otherwise impossible to produce.
Norman Noble leads they way in athermal laser micro-machining
To support its original equipment manufacturer (OEM) customers, medical device contract manufacturer Norman Noble developed Noble S.T.E.A.L.T.H. (System To Enable Ablation Laser Technology Haz-Free), an exclusive, athermal laser machining process that represents an advancement of several magnitudes in medical device production.
Noble S.T.E.A.L.T.H. makes it possible to produce next-generation bioabsorbable scaffolds and Nitinol-based micro implant designs that could not previously be manufactured using current technologies.
Noble S.T.E.A.L.T.H. is an ultrashort pulse (USP) proprietary laser system integrated by Norman Noble, to create highly precise features in any material, such as bioabsorbable polymers, shape memory metals (Nitinol) and other exotic alloys without producing any heat affected zone (HAZ). It can reduce, and in some cases, eliminate costly deburring and post-processing steps, which increases product quality and yield to previously unattainable levels and leaves the machined geometry intact. Accordingly, the Noble S.T.E.A.L.T.H. produces the narrowest laser-cut kerfs in the industry at kerf widths of 0.00045 in. in Nitinol and 0.00025 in. in bioresorbable materials.
In addition to enabling production of new designs, Noble S.T.E.A.L.T.H. provides increased flexibility within existing designs, which can then be manufactured three-times faster than they can be with current laser machining technologies.
Benefits of partnering with Norman Noble on athermal laser machining include:
• Heat-free machining of bioabsorbable materials and Nitinol
• Machining that does not produce HAZ
• Reduction or elimination of costly deburring and post-processing steps
• The industry’s fastest laser ablation processing speed
• Increased product quality and yield
Another critical benefit of partnering with Norman Noble for athermal laser machining applications is the company’s dedicated process development centers. These process development centers (PDCs) mirror Norman Noble’s production manufacturing environment, but feature dedicated equipment and teams to assist its customers with product design and development.
Within its PDCs, Norman Noble can:
• Offer immediate “Design For Manufacturability” feedback upon receipt of an RFQ
• Prototype the designs with samples available in days
• Fully develop the process so it is validation-ready when transferred to production equipment
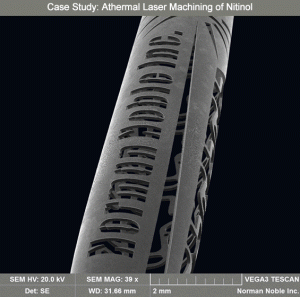
Case Study: Noble S.T.E.A.L.T.H. does what thermal lasers cannot
A medical device OEM presented Norman Noble with an opportunity to manufacture a tubular-based neurovascular implant device. The OEM had manufacturing capabilities in-house, but was not able to produce acceptable product due to capability limitations.
Before approaching Norman Noble, the OEM engaged several local laser contract manufacturers and found little success. Other suppliers using thermal lasers had competitive feedrate issues, ID dross cleaning issues, backwall strikes and oversized kerf widths that exceeded the 0.0005 in. maximum requirement, measuring 0.0011 in. and greater, prohibiting to the design intent. The project was slated for cancellation until the design engineer presented the case for athermal laser machining.
Using its S.T.E.A.L.T.H. athermal laser technology, Norman Noble achieved a kerf width of 0.00045 in. with faster feedrates than traditional laser systems, no backwall strikes and no ID dross. The0 .00045 in. kerf width also allowed the design engineer to make some features smaller than previous specifications because there was no secondary finishing required to remove head damage, which would have enlarged the cut path geometry.
Noble S.T.E.A.L.T.H. enabled the medical device OEM to launch the product and do it in record time. The neurovascular implant device is now considered the standard of care in its product segment.
Noble S.T.E.A.L.T.H. Applications
• Heart valves (Nitinol)
• Stents (Nitinol)
• Bioresorbable scaffolds
• Neurovascular implants and devices (Nitinol and Bioresorbable Materials)
• Vena Cava filters (Nitinol)
Norman Noble
www.nnoble.com

