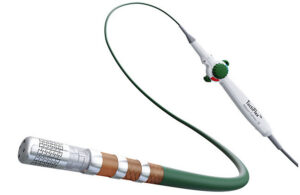
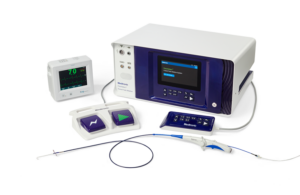
For Abbott, combining two innovations into one AFib-treating system required manufacturing fine-tuning. The project’s R&D director explains. Pulsed field ablation (PFA) has generated a great deal of buzz for its potential to reduce complications in procedures for treating atrial fibrillation (AFib). But even as Abbott is developing its Volt … [Read more...] about How Abbott created its next-gen RF ablation system
Medtronic has a serious catheter tubing recall
The FDA today designated a Medtronic recall of Duet external drainage and monitoring system catheter tubing as Class I, its most serious level. Medtronic Neurosurgery initiated the recall on Jan. 22. According to the FDA, it involves 45,176 devices distributed from May 3, 2021, to Jan. 9, 2024. The model numbers involved are 46913, 46914, 46915, … [Read more...] about Medtronic has a serious catheter tubing recall
ICU Medical’s Smiths Medical has a major syringe pump recall
The FDA this week said it's designated an ICU Medical and Smiths Medical recall of Medfusion syringe pumps as Class I, the agency's most serious level of medical device recall. The recall involves more than 50,000 Medfusion Model 4000 syringe infusion pumps in the U.S. that were distributed from Nov. 16, 2010, to July 28, 2023. The Medfusion … [Read more...] about ICU Medical’s Smiths Medical has a major syringe pump recall
FastWave Medical kicks off first-in-human study of its IVL tech
FastWave Medical announced the successful completion of enrollment for its first-in-human study of its peripheral intravascular lithotripsy (IVL) system to treat calcified cardiovascular disease. Dr. Miguel Montero-Baker of Houston Methodist Hospital and the Hope Vascular & Podiatry Clinic and Dr. Venkatesh Ramaiah of HonorHealth Vascular … [Read more...] about FastWave Medical kicks off first-in-human study of its IVL tech
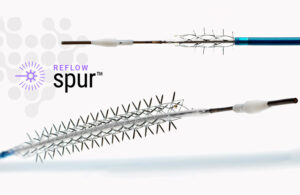
Reflow Medical wins CE mark for temporary spur stent
Reflow Medical announced today that it has received a CE mark in the EU for its Bare Temporary Spur Stent System. The system treats de novo or restenotic lesions in the infrapopliteal arteries, followed by a commercially available drug-coated balloon, to enhance drug absorption. The goal is to provide stent-like results while leaving no metal … [Read more...] about Reflow Medical wins CE mark for temporary spur stent
Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Cook Medical recently announced that its Slip-Cath Beacon Tip hydrophilic selective catheter is once again available in the United States and Canada. This reintroduction follows a collaborative effort with medical professionals to refine the product to meet clinical needs better. The Slip-Cath is designed for vascular and non-vascular … [Read more...] about Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Zeus owners are selling to private equity
The EQT X fund will become the owner of medical tubing tech provider Zeus under an agreement announced today. Financial terms of the acquisition were not disclosed. Bloomberg reported that the deal values the company at $3.4 billion, citing "people with knowledge of the matter." The transaction is expected to close in Q1 2024, subject to … [Read more...] about Zeus owners are selling to private equity
Microbot Medical is a step closer to an FDA IDE submission
Microbot Medical today announced the successful completion of its pivotal pre-clinical study of its Liberty endovascular robotic surgical system. The study, performed under good laboratory practice (GLP) and essential for the company's investigational device exemption (IDE) submission, took place under rigorous FDA guidelines. The study involved … [Read more...] about Microbot Medical is a step closer to an FDA IDE submission
VVT Medical announces first commercial ScleroSafe case in U.S.
VVT Medical recently announced the successful completion of the first commercial U.S. varicose vein treatment using its ScleroSafe platform. Dr. Steve Elias performed the procedure at the Center for Vein Disease, part of the Englewood Health Network in Englewood, New Jersey. “The Sclerosafe procedure takes less than 30 minutes, requires only … [Read more...] about VVT Medical announces first commercial ScleroSafe case in U.S.
Medtronic pulsed-field ablation system wins CE mark
Medtronic said today that it's received a CE mark for its PulseSelect pulsed field ablation system. In addition, the medtech giant received European Union approval for its Nitron CryoConsole, which builds upon the legacy of the company's cryo franchise with features to optimize the workflow for cryoballoon ablation. The news of the regulatory … [Read more...] about Medtronic pulsed-field ablation system wins CE mark