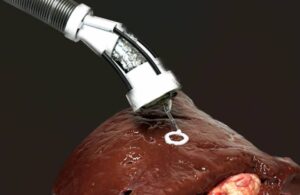
The FDA issued Olympus a warning letter regarding adultered devices following an inspection of the company’s Tokyo facility. This marks the latest warning letter sent by the FDA to Olympus. The company received two separate letters at the end of last year. According to the letter, the agency conducted an inspection of the Tokyo facility … [Read more...] about FDA sends warning letter to Olympus over endoscope manufacturing
Endoscopes
This surgical robotic arm could 3D bioprint inside the human body
Engineers in Australia say they have developed a miniature robotic arm for 3D printing biomaterial directly on human organs. UNSW Sydney researchers developed the device as a flexible, soft robotic arm for 3D bioprinting. This process fabricates biomedical parts from “bioink” to construct natural, tissue-like structures. Predominantly used for … [Read more...] about This surgical robotic arm could 3D bioprint inside the human body
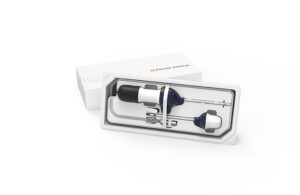
FDA clears single-use endotherapy device from Lumendi
Lumendi today announced it received FDA 510(k) clearance for two of its single-use, disposable endotherapy devices. The Westport, Connecticut-based company received FDA clearance for its DiLumen EZ¹ single-use, disposable end-therapy device for endoscopic mucosal resections and difficult colonoscopies. Its DiLumen C¹ was also cleared for use in … [Read more...] about FDA clears single-use endotherapy device from Lumendi
FDA hits Olympus with warning letters after factory inspections
The FDA today released warning letters against Olympus Medical Systems Corp. and Olympus Corp. subsidiary Aizu Olympus following inspections of their endoscope and reprocessor manufacturing facilities. The warning letters allege medical device reporting (MDR) and quality system violations at the manufacturing operations. “Olympus’ highest … [Read more...] about FDA hits Olympus with warning letters after factory inspections
FDA clears Pristine Surgical single-use arthroscope
Pristine Surgical announced today that FDA has cleared its Summit 4K single-use surgical arthroscope Manchester, New Hampshire–based Pristine describes the arthroscope as a first of its kind. It plans to launch Summit during the first quarter of this year. Summit combines a 4K single-use surgical arthroscope with the company's cloud-based … [Read more...] about FDA clears Pristine Surgical single-use arthroscope
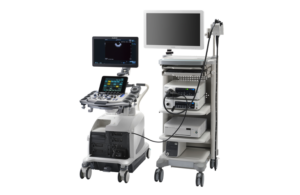
Fujifilm wins FDA clearance for endoscopic ultrasound scope with Arietta 850 processor
Fujifilm recently announced it received FDA 510(k) clearance for its EG-740UT1 linear endoscopic ultrasound scope that is compatible with its Arietta 8502 ultrasound processor. The company designed EG-740UT for diagnostic and therapeutic endoscopic ultrasound procedures in the upper digestive tract, including the esophagus, stomach and duodenum. … [Read more...] about Fujifilm wins FDA clearance for endoscopic ultrasound scope with Arietta 850 processor
Imagin Medical acquires Trod Medical’s enCage Coil precision ablation system
Imagin Medical this week announced it has acquired Trod Medical's enCage Coil precision ablation system. The company will acquire the enCage Coil for $2.5 million, which will be paid over time once certain developmental milestones are met. There will be an initial payment on the closing of $350,000 and $150,000 of Imagin shares. Of the initial … [Read more...] about Imagin Medical acquires Trod Medical’s enCage Coil precision ablation system
Endo Tools Therapeutics announces first commercial cases of Endomina endoscopic suture device
Endo Tools Therapeutics (ETT) this week announced the first U.S. commercial cases using its Endomina system. Gosselies, Belgium-based ETT designed Endomina for endoscopic placement of sutures and approximation of soft tissue in the gastrointestinal tract. The first procedures were performed at Brigham and Women's Hospital in Boston, … [Read more...] about Endo Tools Therapeutics announces first commercial cases of Endomina endoscopic suture device
EndoQuest Robotics’ endoluminal surgical robot wins best application award in 2022 Surgical Robotic Challenge
EndoQuest Robotics today announced its Endoluminal Surgical (ELS) system won the best application award in the 2022 Surgical Robotic Challenge. The Surgical Robotic Challenge is an international competition for healthcare robotics groups to showcase new and innovative ideas across a range of surgical robot platforms. It is presented at the … [Read more...] about EndoQuest Robotics’ endoluminal surgical robot wins best application award in 2022 Surgical Robotic Challenge
The 24 best medical device innovations of 2022
The Galien Foundation recently announced its nominees of medical device innovations for its 2022 Prix Galien USA awards. There are 24 medical technologies nominated for the annual award this year, up from 18 nominees in 2021. The Galien Foundation’s annual Prix Galien awards highlight devices, biotechnology and pharmaceutical products … [Read more...] about The 24 best medical device innovations of 2022