Terumo Interventional Systems announced today that it received FDA 510(k) clearance for its OpusWave dual-sensor imaging system. OpusWave features the DualView imaging catheter, which received FDA 510(k) clearance alongside the entire system. The platform combines optical frequency domain imaging (OFDI) and intravascular ultrasound (IVUS). It aims … [Read more...] about Terumo enters U.S. imaging market with FDA nod for dual-sensor system
Catheters
Merit Medical to acquire cryoballoon tech from Pentax
Merit Medical Systems (Nasdaq:MMSI) announced an agreement to acquire the C2 CryoBalloon device from a Pentax Medical subsidiary. South Jordan, Utah-based Merit signed a definitive asset purchase agreement with Pentax of America over the C2 CryoBalloon and related technology. The companies expect the closing of the deal to occur during the fourth … [Read more...] about Merit Medical to acquire cryoballoon tech from Pentax
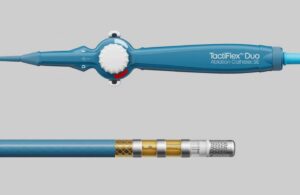
Abbott gets FDA breakthrough nod for dual-energy ablation catheter
An Abbott [WtwhTicker symbol="ABT"](NYSE: ABT)[/WtwhTicker] official posted on social media to announce FDA breakthrough device designation for a new ablation catheter. Abbott SVP Uri Yaron said on LinkedIn that the FDA granted the breakthrough nod for the TactiFlex Duo Sensor-Enabled catheter. The catheter features a dual-energy modality for the … [Read more...] about Abbott gets FDA breakthrough nod for dual-energy ablation catheter
Johnson & Johnson MedTech launches ventricular arrhythmia ablation registry
Johnson & Johnson MedTech today introduced the Collaborative Outcomes Registry for Evidence in Ventricular Arrhythmias (CORE-VA). The company announced the registry during the International Symposium on Ventricular Arrhythmias in Philadelphia. It aims to capture contemporary practice patterns in ventricular arrhythmia (VA). That includes … [Read more...] about Johnson & Johnson MedTech launches ventricular arrhythmia ablation registry
Pulse Biosciences shares strong first-in-human PFA data
Pulse Biosciences (Nasdaq:PLSE) today announced clinical study results from a first-in-human study of its nPulse system. The company shared findings from the first-in-human feasibility study of the cardiac surgical system at the 39th European Association for Cardio-Thoracic Surgery Annual Meeting in Copenhagen, Denmark. Pulse Biosciences' … [Read more...] about Pulse Biosciences shares strong first-in-human PFA data
BD enrolls first patient in Rotarex catheter registry
BD [WtwhTicker symbol="BDX"](NYSE: BDX)[/WtwhTicker] today announced the enrollment of the first patient in the XTRACT Registry for its Rotarex catheter system. The prospective, multi-center, single-arm, post-market registry evaluates Rotarex's real-world performance in treating patients with peripheral artery disease (PAD) lesions. Franklin … [Read more...] about BD enrolls first patient in Rotarex catheter registry
Medtronic launches full U.S. distribution of Neuroguard IEP carotid stent system
Medtronic today announced the full U.S. rollout of the Neuroguard IEP system, a carotid stent and embolic protection device. The launch follows a limited market release and builds on Medtronic’s exclusive U.S. distribution agreement with the device’s developer Contego Medical. Neuroguard IEP combines a nitinol stent, dilation balloon and … [Read more...] about Medtronic launches full U.S. distribution of Neuroguard IEP carotid stent system
Jupiter Endovascular raises more than $40M in oversubscribed Series B
Jupiter Endovascular announced today that it closed an oversubscribed Series B financing that surpassed its target of $40 million. Sonder Capital led the financing, while Senvest Management, LB Investment and a new strategic corporate investor participated. Menlo Park, California-plans to use proceeds to complete its ongoing SPIRARE II pivotal … [Read more...] about Jupiter Endovascular raises more than $40M in oversubscribed Series B
Elixir Medical announces full LithiX IVL rollout in Europe
Elixir Medical announced the full market release of its LithiX intravascular lithotripsy (IVL) system in Europe. The Milpitas, California–based company kicked off its initial rollout for the IVL system in Europe following CE mark in April. Now, it has fully launched the advanced hertz contact (HC)-IVL system in the region. Elixir designed LithiX … [Read more...] about Elixir Medical announces full LithiX IVL rollout in Europe
Adagio completes enrollment in ultra-low temp cryoablation trial
Adagio Medical (Nasdaq:ADGM) announced today that it completed enrollment for its FULCRUM-VT pivotal FDA IDE study. FULCRUM-VT evaluates the company's vCLAS cryoablation system for the ablation of monomorphic ventricular tachycardia (MMVT). The Laguna Hills, California–based company built its vCLAS cryoablation catheter on its proprietary … [Read more...] about Adagio completes enrollment in ultra-low temp cryoablation trial