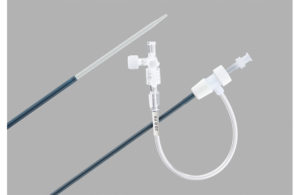
Cook Medical has launched a voluntary recall of nearly 59,000 devices intended to introduce therapeutic or diagnostic devices into the vasculature, excluding coronary and neuro vasculature.
The Flexor Check-Flo introducer and Flexor Tuohy-Borst side-arm introducer (Shuttle Select) are sterile, single-use devices that incorporate a hydrophilic-coated shaft in varying stiffnesses with distal radiopaque markers. The recall covers specific lots of 4-9 Fr Flexor Check-Flo Introducers (high-flex Ansel) and 5 & 6 Fr Flexor Shuttle Select introducers, totaling 58,499 units globally, according to the recall notice.
Cook has received 9 reports of sheath separation during use, which could result in life-threatening adverse events, the notice said. Potential adverse events include, but are not limited to, increased procedural time, intervention to retrieve a separated segment, embolization occluding blood flow to a vital organ, vessel injury and hemorrhage.
Cook began the recall “out of an abundance of caution and support for physicians and patients,” said company spokesperson Hannah Chudleigh in an email to Medical Tubing + Extrusion. “There have been 4 reports of intervention to retrieve a separated segment. There have been no reports of injury such as embolization occluding blood flow to a vital organ, vessel injury, hemorrhage or patient death.”

