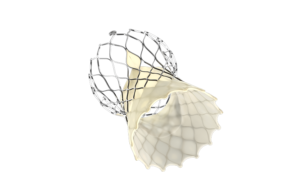
 Medtronic today announced that its Evolut FX TAVR system has won FDA approval.
Medtronic today announced that its Evolut FX TAVR system has won FDA approval.
Fridley, Minn.–based Medtronic designed the self-expanding transcatheter aortic valve replacement system to treat symptomatic severe aortic stenosis.
The TAVR system uses a supra-annular valve design that has demonstrated hemodynamic performance that is superior to surgical aortic valve replacement in large-scale, randomized clinical trials, according to the company. In addition, it has gold markers built into the frame to provide direct visualization of depth and valve leaflet location during an implant.
Evolut FX has a redesigned catheter tip for a smoother insertion profile and a more flexible delivery system that has 360º freedom of motion with stable and predictable deployment. Like Medtronic’s Evolut Pro+, the Evolut FX has four valve sizes for a large indicated patient treatment range and a low delivery profile.
“The self-expanding, supra-annular Evolut platform has evolved considerably over time and has brought heart teams innovative features like recapturability, an expanded size matrix, and advanced valve sealing to help minimize paravalvular leak. Today, the Evolut FX system further refines a trusted platform with key product and procedural enhancements that make the self-expanding system easier to use with enhanced visualization capabilities for orientation and depth,” said Dr. Jeffrey Popma, VP and chief medical officer for Medtronic’s coronary and renal denervation business and the structural heart and aortic business.
Medtronic is planning a limited commercial release in the fall and a full device launch in early 2022.

