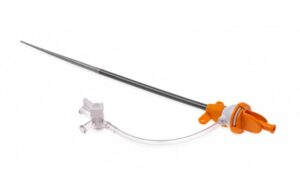
Danvers, Massachusetts-based Abiomed designed its sheath to maintain the same inner diameter as the existing 14 French (Fr) sheath used for Impella CP placement. However, the new sheath reduces the outer diameter by nearly 2 Fr.
The sheath’s smaller size, along with other technological advancements, facilitates easier Impella insertion and removal. Abiomed said in a news release that it helps to reduce procedural steps and help improve outcomes.
Abiomed designed the low-profile sheath for compatibility with the Impella single-access technique. This technique removes the need for an additional access site.
The company said the sheath eliminates the peel-away sheath and the need for the re-access sheath in patients sent to the ICU. This simplifies access and ease of use, Abiomed noted. Additionally, the sheath minimizes vascular complications and bleeding.
Abiomed said it offers easier delivery into the vasculature with a hydrophilic-coated long-taper dilator. This reduces the need for multiple steps of serial dilation. The sheath also helps to facilitate easier patient management during heart pump removal during vascular closure. It allows the direct removal of Impella from the sheath without re-wiring.
The company plans to begin a phased rollout for the low-profile sheath this quarter.
“Abiomed’s Low Profile Sheath is a game-changing technological achievement that will further improve patient outcomes by making it even easier for physicians to insert, manage and remove Impella heart pumps,” said Dr. Chuck Simonton, Abiomed CMO.

