Company researchers developed cardiac ablation technology that mimics the navigation system of electric fish.
Scientists have studied electric fish for decades to determine how and why they discharge electricity.
Recently, a couple of biomedical engineers at Boston Scientific realized that the way these fish use their electrical impulses to navigate around hazards could apply to treating atrial fibrillation (AF).
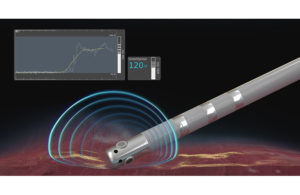
The result is DirectSense technology, which the company uses in its Intellanav MiFi open-irrigated (OI) ablation catheter to measure local impedance — or the amount of resistance — in the tissue to the electrical current that the catheter is applying.
Here’s how ablation works: One cause of atrial fibrillation is heart tissue that extends into one of four pulmonary veins that travel from the lungs to deliver oxygenated blood. This tissue can be irritable, releasing chaotic electrical signals that reach the upper chambers of the heart and cause an abnormal rhythm. Cardiac ablation scars or destroy that tissue and restores normal heart rhythm.
In the past, electrophysiologists looked at the mechanical connection of catheter to tissue in order to apply radiofrequency (RF) pulses to destroy tissue that they believed was causing the irregular heartbeat. However, they have never had a direct measure of the electrical coupling of catheter to tissue, which provides important insights, according to Dr. Kenneth Stein, SVP and CMO for Rhythm Management and Global Health Policy at Boston Scientific.
Three miniature electrodes near the distal tip of the Intellanav catheter provide data on the impedance around the catheter tip to measure the tissue’s ability to respond to RF energy before physicians deliver therapy. During ablation, DirectSense tracks the change in local impedance which, in conjunction with other measures, helps physicians understand tissue characteristics and how they are affecting that tissue. These insights may indicate temperature change in the tissue, helping to reduce the chances of over-ablation and avoid complications.
Boston Scientific researchers did extensive modeling and preclinical experiments and some very detailed human trials to determine if the data produced by DirectSense were meaningful, according to Stein. Data from a retrospective analysis of the technology, presented at Heart Rhythm Society 2020 Science, revealed a local impedance decrease of ≥16.6 ohms with an inter-lesion spacing of ≤ 6mm and a ≥ 98% positive predicative value of durable pulmonary vein block at three months in patients with AF.
In other words, if the spacing is less than 6 mm apart every time you move the ablation catheter, you can have a 98% assurance that you have a complete ablation with no gaps, Stein explained.
The durability of the ablation lesions is critical to the procedure’s long-term success. “This is a safe procedure. It’s an effective procedure,” he added. “(But) no one wants to undergo it twice.”
Boston Scientific launched DirectSense in the EU in 2018 and gained FDA approval for the technology in April 2020. The company waited until June 1, 2020 to launch in the U.S. because so few people were undergoing elective procedures during the COVID-19 pandemic. The reception among physicians has been positive, according to Stein.
Boston Scientific even made a video about how the technology was developed. In it, research scientist Jake Laughner explained how specialized cells in electric fish enable them to detect hazards.
“Electric fish use electrophysiology to actually navigate every single day,” Laughner said. “Why shouldn’t we be using this really elegant system and apply this to our catheters?”
“What we’re really doing here is giving physicians a little bit of light in a dark room,” added fellow research scientist Matt Sulkin. “It makes their jobs just a little bit easier.”

