Medtronic has issued a warning letter regarding some of its Perfusion Tubing Packs.
Medtronic has issued a warning letter regarding some of its Perfusion Tubing Packs.
Friedly, Minnesota-based Medtronic’s urgent field safety notice informs users that some of its Perfusion Tubing Packs were manufactured without device-level testing for bacterial endotoxin, which is a necessary step to support non-pyrogenic label claim. The potential patient harms from endotoxin-mediated pyrogenicity include fever, infection, toxic reaction and death from complete organ failure.
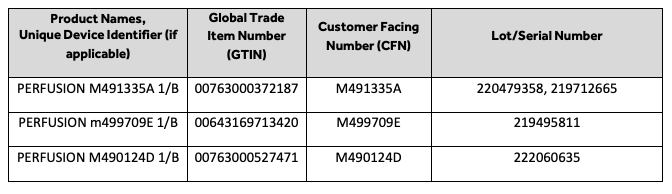
Medtronic said in the notice posted in Germany that an evaluation of existing manufacturing controls and historical data suggest that the devices are still likely to meet the requirement to be non-pyrogenic, but due to the lack of data required to support the label claim, the company has decided to recall 165 lots of the Perfusion Tubing Packs.
The company has received no complaints or reports of adverse events related to the issue to date.
Medtronic is asking users to review inventories for its Perfusion Tubing Packs affected by the issue, quarantine all unused products and return them to the company.
The affected lots include: