Catheter-based devices had their year in 2023. Medtech companies set their sights on improving patient care in cardiology, neurology and more. Our previous roundup of the top 10 most-read catheter-based news stories of 2023 wasn't enough for us. Medical Tubing + Extrusion's editors could not stop thinking about several other stories when … [Read more...] about Catheter-based innovations we can’t stop thinking about
Endolumik
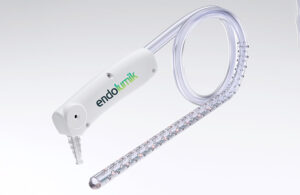
Endolumik’s illuminated device takes a big step for safety
Endolumik's story started a few years ago as the FDA sounded the alarm over the risks of internal surgical staplers. In 2019, the federal agency warned healthcare providers that it had received more than 41,000 medical device reports (MDRs) related to surgical staplers and staples for internal use from 2011 to 2018. Those MDRs tallied more than … [Read more...] about Endolumik’s illuminated device takes a big step for safety
FDA clears first device authorized under Safer Technologies Program
The FDA last week cleared a new gastric calibration tube, marking the first authorization of a device under the agency's Safer Technologies Program. FDA launched its Safer Technologies Program in 2021. It modeled the program on its breakthrough devices program. The Safer Technologies Program (STeP) covers devices that could improve the safety of … [Read more...] about FDA clears first device authorized under Safer Technologies Program