The FDA shared an alert that BD (NYSE: BDX) and its Bard subsidiary warned customers of an issue with certain intravascular catheters. BD recommended the removal of certain unused PowerPICC intravascular catheters from use or sale. In-use PowerPICC intravascular catheters also received updated instructions for use. Affected products include the … [Read more...] about BD warns on intravascular catheter
fda
Conavi warns on intravascular catheter issue
The FDA today issued an alert related to a potentially high-risk device issue with certain Conavi Novasight Hybrid catheters. Its communication comes as part of its pilot to enhance the medical device recall program. The FDA said it became aware of the issue after Conavi sent a letter to affected customers recommending the removal of certain … [Read more...] about Conavi warns on intravascular catheter issue
Abbott is making an IVL play with FDA IDE nod
Abbott announced today that it received FDA investigational device exemption (IDE) approval for its coronary intravascular lithotripsy (IVL) system. IDE enables the company to evaluate the system in treating severe calcification in coronary arteries prior to stenting. The company expects its TECTONIC coronary artery disease (CAD) IVL trial to … [Read more...] about Abbott is making an IVL play with FDA IDE nod
Medtronic recalls embolization device after reported deaths
The FDA deemed a recall of some Medtronic Pipeline Vantage embolization devices serious after multiple deaths related to the device. The recall involves removing Pipeline Vantage 027 device models from use and sale and updating instructions for Pipeline Vantage 021 models. Medtronic's Pipeline Vantage embolization devices with Shield Technology … [Read more...] about Medtronic recalls embolization device after reported deaths
Lungpacer wins FDA IDE for AeroNova system
Lungpacer Medical announced today that it received FDA investigational device exemption (IDE) to begin a trial for its AeroNova system. The STARI (stimulation to activate respiration) trial evaluates the feasibility of the investigational AeroNova system. It looks at the system in patients suffering from moderate to severe Acute Hypoxemic … [Read more...] about Lungpacer wins FDA IDE for AeroNova system
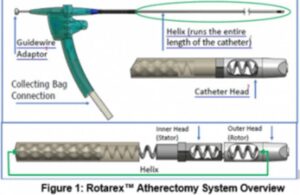
BD warns on atherectomy catheters after deaths
BD (NYSE: BDX) recently issued a medical device correction later related to its Bard subsidiary's Rotarex atherectomy system. The company identified a number of anatomical factors that could contribute to catheter helix fracture and/or breakage. It reports 30 serious injuries and four deaths associated with the issue. Additionally, BD reported … [Read more...] about BD warns on atherectomy catheters after deaths
Boston Scientific updates instructions for cryoablation catheters after reported deaths
The FDA issued a notice alerting customers to updates made by Boston Scientific to its PolarX cryoablation catheters. This recall involves updating instructions for use (IFU), not removing devices from the market. The FDA identified it as the most serious type of recall, though, as it may cause serious injury or death. Boston Scientific … [Read more...] about Boston Scientific updates instructions for cryoablation catheters after reported deaths
FDA approves IDE for Akura Medical’s thrombectomy system
Akura Medical, a Shifamed portfolio company, announced today that the FDA granted its Katana system investigational device exemption (IDE). IDE means Akura can begin the QUADRA-PE study evaluating the thrombectomy system in patients with acute pulmonary embolism (PE). Dr. Sanjum Sethi (Columbia University Medical Center) and Dr. Ann Gage … [Read more...] about FDA approves IDE for Akura Medical’s thrombectomy system
R3 Vascular wins FDA IDE for drug-eluting scaffold
R3 Vascular announced today that the FDA granted investigational device exemption (IDE) to evaluate its Magnitude drug-eluting bioresorbable scaffold. Mountain View, California-based R3 Vascular designed Magnitude for treating below-the-knee (BTK) peripheral arterial disease (PAD). With the FDA granting IDE, it can now initiate the ELITE-BTK … [Read more...] about R3 Vascular wins FDA IDE for drug-eluting scaffold
Vantis Vascular wins FDA nod for integrated microcatheter guide extension system
Vantis Vascular announced today that it received FDA 510(k) clearance for its CrossFast integrated microcatheter guide extension system. San Jose, California-based Vantis develops the CrossFast system along with the CrossShock intravascular lithotripsy (IVL) system. It designed CrossFast to help physicians perform faster, easier and safer … [Read more...] about Vantis Vascular wins FDA nod for integrated microcatheter guide extension system