Vascular Solutions: Langston dual lumen catheter
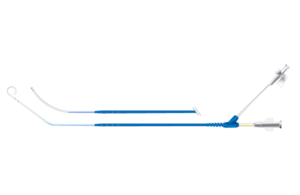
The recall involved 4,304 devices, which are used for the rapid delivery of contrast material into a patient’s blood vessels during medical imaging tests to allow clinicians to see internal body structure. The devices pose the risk of the inner catheter separating during use, which can cause serious health conditions, including additional surgical procedures, damage to the blood vessel or death.
Additionally, if the inner catheter separates outside of the patient’s body, the doctor could be sprayed by the dye, leading to an infection that may lead to the need for the doctor to receive treatment.
There have been eight complaints and no reports of injury or death, according to an April 30 FDA warning.
Vascular Solutions urged customers to secure and remove all unused affected devices. Teleflex intended to destroy any unused recalled devices.
Catheters involved in the recall were made between June 22, 2019, and Dec. 2, 2019. They were distributed between July 12, 2019, and March 10, 2020.

