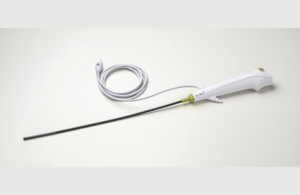
 Ambu today said it received Health Canada clearance for its aScope 4 Cysto single-use cystoscope.
Ambu today said it received Health Canada clearance for its aScope 4 Cysto single-use cystoscope.
The cystoscope is a flexible, single-use endoscope that is designed to eliminate significant capital, repair and cleaning costs with a patient-ready instrument for every procedure, according to Ambu. The device is used for diagnosing, managing and treating lower urinary disorders such as hematuria, incontinence and bladder cancer.
“We are excited to bring our single-use cystoscope to urologists in Canada,” Ambu president Steven Block said in a news release. “Since launching in the United States, we have seen the positive impact our technology can have – redefining productivity for the doctors, staff, and the facility, while ensuring a safe, sterile device for the patient.”
The aScope 4 cystoscope is sterile from the package and ready when it is needed. It uses the company’s aView 2 Advance high-definition display for video endoscopy, which is a compact and portable unit that can be easily transported throughout a hospital.
“We know there are challenges in today’s urology clinics – such as COVID-19-created procedure backlogs – that the aScope 4 Cysto can directly address,” Ambu Canada’s national sales director Doug Pedersen said. “By providing a new paradigm for increasing outpatient throughput that isn’t constrained by reprocessing or capital budgets, we can improve urology clinic workflows and patient scheduling flexibility.”

