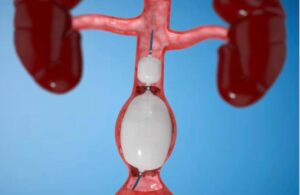
Concept Medical announced today that it commenced the FDA investigational device exemption (IDE) study of its MagicTouch system. MagicTouch, a sirolimus drug-coated balloon (DCB), treats in-stent restenosis (ISR) in coronary artery disease (CAD). Dr. Said Ashraf enrolled the first patient in the MAGICAL-ISR study at the AtlantiCare Institute in … [Read more...] about Concept Medical enrolls first patient in drug-coated balloon study
Balloons
Getinge wins European approval for covered stent system
Getinge announced today that it received EU MDR certification for its Advanta V12 covered stent system for patients with aortoiliac occlusive disease (AIOD). The balloon-expandable covered stent's indication aligns with AIOD, which includes treating lesions at the aortic bifurcation. Getinge said EU MDR certification highlights its dedication … [Read more...] about Getinge wins European approval for covered stent system
Nectero Medical closes $96M Series D
Nectero Medical announced today that it closed a Series D financing round worth proceeds of $96 million. Norwest Venture Partners led the round. Boston Scientific, BioStar Capital, Cadence Healthcare Ventures, Aphelion Capital and other firms contributed large investments. Funds add to a $19.5 million Series C — for which Boston Scientific led … [Read more...] about Nectero Medical closes $96M Series D
Concept Medical wins FDA IDE for MagicTouch AVF drug-coated balloon
Concept Medical today announced it received FDA investigational device exemption for its MagicTouch AVF Sirolimus drug-coated balloon (DCB) catheter. The IDE allows the Tampa, Florida-based company to start a clinical study to treat stenotic lesions of arteriovenous fistula (AVF) in the hemodialysis management of chronic renal failure. "This … [Read more...] about Concept Medical wins FDA IDE for MagicTouch AVF drug-coated balloon
Boston Scientific has positive Agent DCB study results
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] reported positive investigational device exemption (IDE) trial data for its Agent drug-coated balloon (DCB). The DCB just this month won FDA approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue … [Read more...] about Boston Scientific has positive Agent DCB study results
FDA approves Boston Scientific’s Agent drug coated balloon
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it received FDA approval for its Agent drug-coated balloon (DCB). The DCB won approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented … [Read more...] about FDA approves Boston Scientific’s Agent drug coated balloon
FDA approves Gore low-profile balloon-expandable endoprosthesis
W.L. Gore & Associates announced today that it received FDA approval for a lower-profile Viabahn VBX balloon-expandable endoprosthesis. FDA approval builds on the company's proven VBX stent graft for treating complex vascular disease. The company says it offers the longest balloon-expandable stent on the market (79 mm) and the widest range … [Read more...] about FDA approves Gore low-profile balloon-expandable endoprosthesis
Abbott bets on balloons in pulse field ablation battle
One component stands out as unique when you compare the Abbott Volt against competing pulse field ablation (PFA) systems from Medtronic and Boston Scientific. The Volt cardiac ablation catheter has a balloon, something you won’t see on Medtronic’s PulseSelect PFA catheter — the first of its kind approved by the FDA — or the Farawave catheter in … [Read more...] about Abbott bets on balloons in pulse field ablation battle
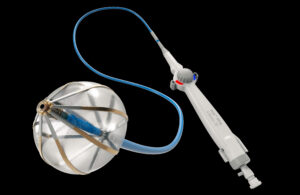
FastWave Medical kicks off first-in-human study of its IVL tech
FastWave Medical announced the successful completion of enrollment for its first-in-human study of its peripheral intravascular lithotripsy (IVL) system to treat calcified cardiovascular disease. Dr. Miguel Montero-Baker of Houston Methodist Hospital and the Hope Vascular & Podiatry Clinic and Dr. Venkatesh Ramaiah of HonorHealth Vascular … [Read more...] about FastWave Medical kicks off first-in-human study of its IVL tech
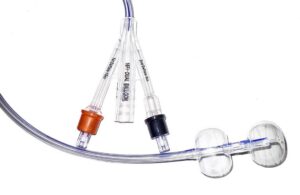
HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical
HR Pharmaceuticals announced that it entered into an exclusive commercial agreement with Poiesis Medical to license catheter technology. The deal centers around Poiesis' dual-balloon catheter, the Duette system. Under the terms, HR Pharmaceuticals has exclusive commercialization rights to Duette in North America. The deal expands HR … [Read more...] about HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical