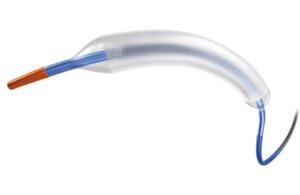
The FDA says a recall of Fresenius Medical Care catheter extension sets and luer lock adapters is Class I, the most serious kind. Fresenius Medical Care initiated the recall of a number of its Stay-Safe luer lock adapters and catheter extensions on Jan. 23, 2024. It extends to nearly 2.2 million devices distributed between March 5, 2003, and … [Read more...] about Fresenius Medical Care recalls more than 2 million catheter components
fda
FDA approves Boston Scientific’s Agent drug coated balloon
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it received FDA approval for its Agent drug-coated balloon (DCB). The DCB won approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented … [Read more...] about FDA approves Boston Scientific’s Agent drug coated balloon
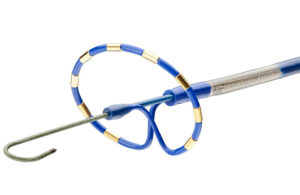
FDA panel votes in favor of Abbott TriClip TEER system for treating tricuspid regurgitation
Abbott (NYSE:ABT) announced today that an FDA advisory committee ruled that the benefits of its TriClip outweigh the risks in treating tricuspid regurgitation (TR). The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the FDA confirmed this with votes registering 13 to 1 in favor of TriClip’s benefits. Abbott … [Read more...] about FDA panel votes in favor of Abbott TriClip TEER system for treating tricuspid regurgitation
Medtronic PulseSelect pulsed field ablation wins FDA approval
The FDA has approved the Medtronic PulseSelect pulsed field ablation (PFA) system, the device developer said today. PulseSelect is the first PFA technology approved for use in the U.S., as well as the first PFA technology with FDA breakthrough designation to win approval. The minimally invasive, cardiac ablation system is indicated for the … [Read more...] about Medtronic PulseSelect pulsed field ablation wins FDA approval
FDA approves LimFlow chronic limb-threatening ischemia treatment
LimFlow announced today that the FDA approved its LimFlow System for treating chronic limb-threatening ischemia (CLTI). Approval enables the treatment to help those with CLTI with no other suitable endovascular or surgical treatment options available. These patients can face major amputation as a result of their condition. France-based … [Read more...] about FDA approves LimFlow chronic limb-threatening ischemia treatment
J&J used RWE for expanded indications — and you can, too
Two J&J MedTech leaders shared advice to help medical device developers use real-world evidence (RWE) in FDA submissions. Real-world evidence (RWE) took a big step forward recently when the FDA approved expanded indications for Johnson & Johnson MedTech ablation catheters. For the first time, the federal medical device safety regulator … [Read more...] about J&J used RWE for expanded indications — and you can, too
FDA approves drug-coated balloon for BPH symptoms from Urotronic
Urotronic announced that the FDA approved its Optilume BPH catheter system for alleviating urinary symptoms caused by BPH. Minneapolis-based Urotronic designed Optilume as a minimally invasive surgical therapy. It combines mechanical dilation using a proprietary double-lobe balloon with concurrent localized delivery of paclitaxel. This treats … [Read more...] about FDA approves drug-coated balloon for BPH symptoms from Urotronic
FDA updates guidance on paclitaxel-coated devices, determines no link to late mortality risk
The FDA issued healthcare providers updated guidance for certain warning language with paclitaxel-coated devices that treat PAD. These peripheral arterial disease (PAD)-treating devices produced data that does not support an excess mortality risk. Specifically, the FDA's guidance eliminates the need for certain warning language in the device … [Read more...] about FDA updates guidance on paclitaxel-coated devices, determines no link to late mortality risk
Route 92 Medical wins FDA clearance for stroke treatment system
Route 92 Medical announced that it received FDA 510(k) clearance for its FreeClimb 70 reperfusion system for treating ischemic stroke. San Mateo, California-based Route 92 designed the reperfusion system with the FreeClimb 70 aspiration catheter. It also features a Tenzing 7 delivery catheter. The system enables physicians to treat patients … [Read more...] about Route 92 Medical wins FDA clearance for stroke treatment system
Surmodics targets FDA premarket approval for drug-coated balloon in Q4
Surmodics (Nasdaq:SRDX) announced today that it received formal feedback from the FDA related to its SurVeil drug-coated balloon (DCB). Eden Prairie, Minnesota-based Surmodics gave the FDA a proposed approach to submit an amended premarket approval application for SurVeil. In January, the FDA indicated that Surmodics’ SurVeil PMA application is … [Read more...] about Surmodics targets FDA premarket approval for drug-coated balloon in Q4