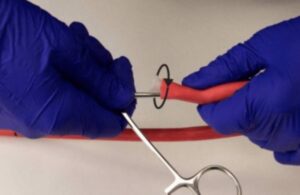
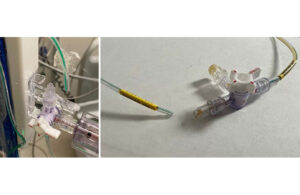
The FDA issued a notice highlighting a letter issued by BD and its C.R. Bard Urology and Critical Care subsidiary warning of new balloon device instructions. This warning follows one death associated with the device issue. BD sent affected customers a notice updating instructions for all lots of certain esophagogastric balloon tamponade tubes. … [Read more...] about BD warns on esophagogastric balloon tamponade tubes after death
Connectors
Fresenius Medical Care recalls more than 2 million catheter components
The FDA says a recall of Fresenius Medical Care catheter extension sets and luer lock adapters is Class I, the most serious kind. Fresenius Medical Care initiated the recall of a number of its Stay-Safe luer lock adapters and catheter extensions on Jan. 23, 2024. It extends to nearly 2.2 million devices distributed between March 5, 2003, and … [Read more...] about Fresenius Medical Care recalls more than 2 million catheter components
Medtronic has a serious catheter tubing recall
The FDA today designated a Medtronic recall of Duet external drainage and monitoring system catheter tubing as Class I, its most serious level. Medtronic Neurosurgery initiated the recall on Jan. 22. According to the FDA, it involves 45,176 devices distributed from May 3, 2021, to Jan. 9, 2024. The model numbers involved are 46913, 46914, 46915, … [Read more...] about Medtronic has a serious catheter tubing recall
Qosina announces new luer lock connectors amid nylon shortage
Qosina (Ronkonkoma, New York) announced today that it is adding to its luer lock connector product line to address a global Zytel nylon shortage. The new selection of luer lock connectors is made of high-performance Vydyne that the company has in stock. As a result, the connectors are ready for immediate shipment. In addition, the connectors are … [Read more...] about Qosina announces new luer lock connectors amid nylon shortage
Biomerics expands Costa Rica, Texas manufacturing operations
Biomerics recently announced that it is doubling its manufacturing footprint in Costa Rica and Texas. The Salt Lake City, Utah-based company broke ground on a new greenfield plant adjacent to its current operations in the La Zeta Free Trade Zone in Cartago, Costa Rica. The new facility totals 110,000 sq. ft and will include engineering labs, … [Read more...] about Biomerics expands Costa Rica, Texas manufacturing operations
This swelling fluid helps boost medical tubing assembly
MicroCare Medical has earned success selling its Swellex silicone swelling fluid to companies assembling medical tubing. Here’s how it works. MicroCare Medical (New Britain, Conn.) over the past decade has found that its Swellex silicone swelling fluid is a popular product for medical tubing manufacturers. “That’s because the swelling fluid … [Read more...] about This swelling fluid helps boost medical tubing assembly
Qosina expands ISO 80369-7 compliant component offerings
Qosina (Ronkonkoma, N.Y.) recently announced that it has expanded its ISO 80369-7 compliant offerings. The ISO certification specifies the dimensions and requirements for the design and performance of small-bore connectors that are designed to be used in intravascular or hypodermic applications. The ISO 80369-7 standard replaces ISO 594-1 and ISO … [Read more...] about Qosina expands ISO 80369-7 compliant component offerings
Qosina adds CPC AseptiQuik G and S series connectors
Qosina (Ronkonkoma, N.Y.) recently announced that it has added its full line of AseptiQuik G and S series connectors to its online offering. The connectors are ideal for easy sterile connections for small-flow applications and can be used in non-sterile environments. They also have a genderless design that allows them to be used universally to … [Read more...] about Qosina adds CPC AseptiQuik G and S series connectors
ODU touts Medi-Snap Advanced plastic circular connector for medical applications
ODU (Camarillo, Calif.) recently announced that it is introducing its Medi-Snap Advanced plastic circular connector to the North American market. ODU describes the Medi-Snap as lightweight and cost-efficient. It's a plastic circular connector with high chemical resistance ideal for medical, industrial and test and measurement applications, … [Read more...] about ODU touts Medi-Snap Advanced plastic circular connector for medical applications
Injectech adds to male/female luers lineup
Injectech (Fort Collins, Colo.) said today that it has started selling male/female luer locks and male/female bond-in luers that meet the ISO 80369-7 standard. The new products join Injectech's existing line of male/female luers. The ISO 80369 series of standards is meant to minimize misconnections between small-bore connectors of different … [Read more...] about Injectech adds to male/female luers lineup