Philips today announced the first procedures in Europe with its recently launched VeriSight Pro 3D ICE catheter. Doctors at the German University Medical Center Mainz and Robert Bosch Hospital in Stuttgart completed the first procedures in Europe after the intracardiac echocardiography (ICE) catheter following its launch across the continent last … [Read more...] about Philips reports first cases for VeriSight Pro 3D ICE catheter in Europe
Cardiac Implants
Aortyx raises $16M for bioresorbable aortic patch
Aortyx announced today that it brought in $16 million (€13.8 million) to support its bioresorbable aortic patch technology. The company expects the funding to help bring the patch to first-in-human tests within two years. Funding came from Ship2B Ventures, through its BSocial Impact Fund (supported by Banco Sabadell, the EIF, and AXIS), and Clave … [Read more...] about Aortyx raises $16M for bioresorbable aortic patch
Philips launches real-time 3D intracardiac imaging catheter in Europe
Philips [WtwhTicker symbol="PHG"](NYSE: PHG)[/WtwhTicker] announced today that it launched its VeriSight Pro 3D intracardiac echocardiography (ICE) catheter in Europe. The Dutch medtech giant designed VeriSight Pro 3D to deliver real-time 3D imaging directly inside the heart. It helps physicians perform procedures with greater clarity and … [Read more...] about Philips launches real-time 3D intracardiac imaging catheter in Europe
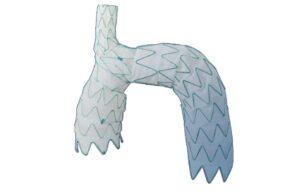
Endospan has positive aortic arch stent graft study results
Endospan today announced the presentation of 30-day results from a study evaluating its Nexus aortic arch stent graft system. Results came out of the statistical dissection primary arm from the TRIOMPHE FDA investigational device exemption (IDE) study. Investigators presented findings at the American Association for Thoracic Surgery (AATS) … [Read more...] about Endospan has positive aortic arch stent graft study results
Supira Medical closes $120M Series E, completes enrollment in ventricular assist device study
Supira Medical announced today that it completed an oversubscribed Series E financing round, bringing in $120 million. The clinical-stage Shifamed portfolio company also recently completed enrollment for its U.S. SUPPORT I Early Feasibility Study (EFS) evaluating its percutaneous ventricular assist device (pVAD) for treating high-risk … [Read more...] about Supira Medical closes $120M Series E, completes enrollment in ventricular assist device study
Abbott reports first cases with software-guided balloon-expandable TAVI
Abbott (NYSE:ABT) today announced the first patient procedures with its investigational transcatheter aortic valve implantation (TAVI) system. The company designed its investigational software-guided, balloon-expandable TAVI system to build a foundation for AI-guided procedures. It hopes to add the expandable system to its structural heart … [Read more...] about Abbott reports first cases with software-guided balloon-expandable TAVI
Merit Medical completes acquisition of Cook Medical’s lead management portfolio
Merit Medical Systems (Nasdaq:MMSI) announced today that it completed its acquisition of Cook Medical’s lead management portfolio. South Jordan, Utah-based Merit announced its agreement to buy the portfolio for $210 million in September. Merit funded the acquisition through a combination of cash on hand and borrowings under a long-term credit … [Read more...] about Merit Medical completes acquisition of Cook Medical’s lead management portfolio
Edwards launches Sapien 3 valve with Alterra prestent in Europe
Edwards Lifesciences (NYSE:EW) announced that it launched its Sapien 3 transcatheter pulmonary valve implant (TPVI) system with Alterra adaptive prestent in Europe. The launch expands the continent’s minimally invasive treatment options to a broader range of patients with congenital heart conditions. Edwards recently received CE mark for … [Read more...] about Edwards launches Sapien 3 valve with Alterra prestent in Europe
Merit Medical to buy Cook Medical’s lead management portfolio
Merit Medical Systems (Nasdaq:MMSI) announced today that it agreed to acquire Cook Medical's lead management portfolio for $210 million. Cook Medical's lead management business provides medical devices and accessories used in lead management procedures. These procedures occur in patients who need a pacemaker or an implantable … [Read more...] about Merit Medical to buy Cook Medical’s lead management portfolio
Biotronik earns expanded FDA nod for pacing lead, delivery catheter system
Biotronik announced today that it received FDA approval for its Selectra 3D catheter and Solia S lead for use in left bundle branch area pacing (LBBAP). The FDA's approval of the new labeling for the pacing combination makes the products the first and only stylet-driven lead and dedicated delivery catheter system approved by the FDA for LBBAP, … [Read more...] about Biotronik earns expanded FDA nod for pacing lead, delivery catheter system