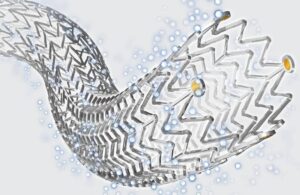
Cook Medical announced today that the U.S. Dept. of Defense (DOD) awarded it an ECAT contract for implantable medical devices. The contract includes implantable vascular medical devices like the Zilver PTX drug-eluting peripheral stent. It also applies to the Zenith aortic endografts and associated interventional devices for treating vascular … [Read more...] about Cook Medical wins DOD contract for drug-eluting stents
Cook Medical
Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Getinge and Cook Medical today announced they signed a commercial distribution agreement for the iCast covered stent system in the U.S. In this deal, Cook Medical will take over sales, marketing, and distribution rights for the product in the U.S. The iCast covered stent system will continue to be manufactured by Atrium Medical, a Getinge … [Read more...] about Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
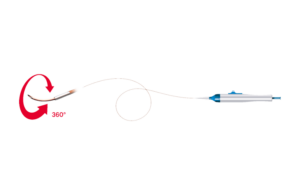
Cook Medical signs distribution agreement with Bentley for BeBack catheter
Cook Medical today announced it signed a U.S. distribution agreement with Bentley for the BeBack catheter. Under the agreement, Cook Medical will assume commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack catheter to our peripheral intervention portfolio,” Alec Cerchiari, director of product … [Read more...] about Cook Medical signs distribution agreement with Bentley for BeBack catheter
Cook Medical’s shorter Liver Access and Biopsy Set wins expanded indication
Cook Medical this week announced its shorter Liver Access and Biopsy Set (LABS) received pediatric indication. LABS was originally intended for use in adults, but was recently FDA cleared for use in adolescents, children and infants. The Bloomington, Indiana-based company designed the device for use in obtaining liver histology samples via … [Read more...] about Cook Medical’s shorter Liver Access and Biopsy Set wins expanded indication
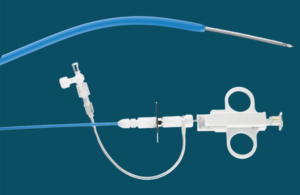
Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Cook Medical recently announced that its Slip-Cath Beacon Tip hydrophilic selective catheter is once again available in the United States and Canada. This reintroduction follows a collaborative effort with medical professionals to refine the product to meet clinical needs better. The Slip-Cath is designed for vascular and non-vascular … [Read more...] about Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
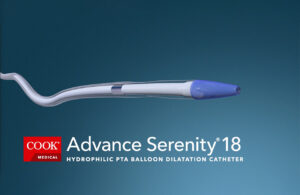
Cook Medical has more hydrophilic PTA balloon catheter options
Cook Medical today announced more sizes and locations for the Advance Serenity hydrophilic PTA balloon catheter product line. It's now possible for U.S. and Canadian interventionalists to use Advance Serenity for below-the-knee and above-the-knee procedures to treat patients with peripheral artery disease (PAD), according to Cook Medical. In … [Read more...] about Cook Medical has more hydrophilic PTA balloon catheter options
Cook Medical, Vizient ink contract for endoscopy devices
Cook Medical announced today that it received a contract with Vizient covering the company's endoscopy devices. The contract enables Cook to continue offering its endoscopy devices at negotiated pricing terms to Vizient members. “We are honored to have been chosen as an Endoscopy contracted supplier with Vizient," said Rick Simms, Cook … [Read more...] about Cook Medical, Vizient ink contract for endoscopy devices
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
Cook Medical wins FDA breakthrough designation for Thoraco+ endovascular system
Cook Medical today announced that its Zenith Thoraco+ endovascular system received FDA breakthrough device designation. Thoraco+ is the company's next-generation endovascular graft that is indicated for the endovascular treatment of patients with thoracoabdominal aortic aneurysms. “We are excited to receive an FDA breakthrough device … [Read more...] about Cook Medical wins FDA breakthrough designation for Thoraco+ endovascular system
Cook Medical wins FDA breakthrough designation for new drug-eluting stent
Cook Medical recently announced that it received FDA breakthrough designation for its drug-eluting stent for below the knee. Bloomington, Indiana-based Cook Medical designed the stent to treat patients who have chronic limb-threatening ischemia (CLTI). "CLTI is a debilitating disease of growing prevalence around the globe and this is Cook … [Read more...] about Cook Medical wins FDA breakthrough designation for new drug-eluting stent