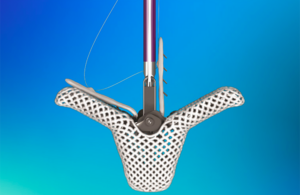
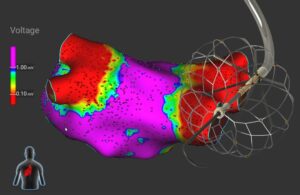
Medtronic recently offered a first look at its Sphere-360 pulsed field ablation (PFA) and mapping catheter, an investigational device with some design features that are new for the world's largest device manufacturer.
In an interview with Medical Design & Outsourcing, Tim Laske, VP of research and business development for Medtronic Cardiac … [Read more...] about First look: Medtronic’s Sphere-360 pulsed field ablation catheter design has some new tricks
Applications
First look: Medtronic’s Sphere-360 pulsed field ablation catheter design has some new tricks
Abbott wins FDA approval for dissolving drug-eluting scaffold
Abbott announced today that the FDA approved its Espirit everolimus-eluting resorbable scaffold system. The Espirit scaffold treats chronic limb-threatening ischemia (CLTI) below the knee (BTK). Abbott designed it to keep arteries open and deliver everolimus to support vessel healing prior to completely dissolving. Abbott says that before … [Read more...] about Abbott wins FDA approval for dissolving drug-eluting scaffold
Roivios wins FDA breakthrough nod for renal assist device
Roivios announced today that it received FDA breakthrough device designation for its JuxtaFlow renal assist device (RAD). The Bahamas-based medical device company designed JuxtaFlow to improve treatments for patients facing kidney disease during cardiac surgery. JuxtaFlow uses a unique, gentle negative pressure technique on the kidneys' … [Read more...] about Roivios wins FDA breakthrough nod for renal assist device
Thermedical completes feasibility study to use SERF with PFA
Thermedical announced today that it completed a feasibility study using pulsed field ablation in combination with its SERF ablation system. Weston, Massachusetts–based Thermedical designed its SERF system and Durablate catheter to work with PFA to treat ventricular tachycardia (VT). According to Thermedical, SERF (saline-enhanced radio … [Read more...] about Thermedical completes feasibility study to use SERF with PFA
Concept Medical enrolls first patient in drug-coated balloon study
Concept Medical announced today that it commenced the FDA investigational device exemption (IDE) study of its MagicTouch system. MagicTouch, a sirolimus drug-coated balloon (DCB), treats in-stent restenosis (ISR) in coronary artery disease (CAD). Dr. Said Ashraf enrolled the first patient in the MAGICAL-ISR study at the AtlantiCare Institute in … [Read more...] about Concept Medical enrolls first patient in drug-coated balloon study
The top catheter-based innovation news stories of 2024 — so far
Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Getinge wins European approval for covered stent system
Getinge announced today that it received EU MDR certification for its Advanta V12 covered stent system for patients with aortoiliac occlusive disease (AIOD). The balloon-expandable covered stent's indication aligns with AIOD, which includes treating lesions at the aortic bifurcation. Getinge said EU MDR certification highlights its dedication … [Read more...] about Getinge wins European approval for covered stent system
Nitinol grips prevent slips in Abbott’s heart valve clips
Nitinol is a key material in the heart valve clips that Abbott designed for its TriClip and MitraClip transcatheter edge-to-edge repair (TEER) systems. Abbott designed the TriClip system (approved by the FDA in April 2024) for reducing tricuspid valve regurgitation using fourth-generation heart valve clips that Abbott originally developed for … [Read more...] about Nitinol grips prevent slips in Abbott’s heart valve clips
Biosense Webster reports positive dual-energy ablation results
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
Medtronic has positive data for single-shot mapping ablation catheter
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced positive clinical trial data for its Sphere-360 investigational pulsed field ablation (PFA) catheter technology. The single-shot mapping and ablation catheter uses pulsed field energy to treat patients with paroxysmal AFib. Medtronic presented interim findings on the … [Read more...] about Medtronic has positive data for single-shot mapping ablation catheter