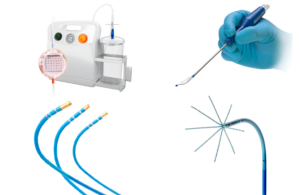
Getinge announced today that it received EU MDR certification for its Advanta V12 covered stent system for patients with aortoiliac occlusive disease (AIOD). The balloon-expandable covered stent's indication aligns with AIOD, which includes treating lesions at the aortic bifurcation. Getinge said EU MDR certification highlights its dedication … [Read more...] about Getinge wins European approval for covered stent system
Getinge
Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Getinge and Cook Medical today announced they signed a commercial distribution agreement for the iCast covered stent system in the U.S. In this deal, Cook Medical will take over sales, marketing, and distribution rights for the product in the U.S. The iCast covered stent system will continue to be manufactured by Atrium Medical, a Getinge … [Read more...] about Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Getinge’s Datascope has its second Class I recall in a month
The FDA issued a notice determining another recall of Getinge subsidiary Datascope’s Cardiosave intra-aortic balloon pumps (IABPs) as Class I, the most serious kind. This recall relates to the Swedish medtech company’s Cardiosave Hybrid and Cardiosave Rescue IABPs. Both devices were subject to a separate Class I recall earlier this month. The … [Read more...] about Getinge’s Datascope has its second Class I recall in a month
Getinge wins FDA PMA for iCast covered stent
Getinge recently announced its iCast covered stent received FDA premarket approval to treat patients with iliac arterial occlusive disease. The iCast covered stent is a clinically-evaluated balloon expandable polytetrafluoroethylene-covered stent. It is delivered using a catheter to hold open and support the walls of structures within the body. … [Read more...] about Getinge wins FDA PMA for iCast covered stent
Getinge’s Datascope has another serious intra-aortic balloon pump recall
The FDA issued a notice determining a recall of Getinge subsidiary Datascope's Cardiosave intra-aortic balloon pumps (IABPs) as Class I, the most serious kind. This recall relates to the Swedish medtech company's Cardiosave Hybrid and Cardiosave Rescue IABPs. Both devices were subject to a separate recall that the FDA determined was Class I in … [Read more...] about Getinge’s Datascope has another serious intra-aortic balloon pump recall
Getinge penalized in EU over life support system problems
The EU notified body DEKRA out of Germany has temporarily suspended the CE certificates for Getinge HLS and PLS sets, starting this month. The Swedish medtech company announced the suspension on Feb. 23. Health providers use the HLS and PLS for extracorporeal respiratory and/or cardiovascular support. Getinge had already announced potential … [Read more...] about Getinge penalized in EU over life support system problems
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
Getinge partners with Medtronic to make Radiant balloon-expandable covered stent
Getinge today announced a partnership with Medtronic (NYSE:MDT) over the Radiant balloon-expandable stent, which received CE mark. Radiant is designed for use in chimney endovascular aneurysm repair (ChEVAR) with the Endurant II/IIs stent graft system. It maintains perfusion to renal arteries when used in combination with the stent graft. The … [Read more...] about Getinge partners with Medtronic to make Radiant balloon-expandable covered stent
Getinge’s Atrium Medical has a Class I recall over covered stents
The FDA today determined that Atrium Medical's recall of some of its iCast covered stents is Class I, the most serious kind. Hudson, N.H.–based Atrium Medical — a subsidiary of Swedish medtech giant Getinge — is recalling certain iCast covered stent systems due to complaints of separation of the balloon or catheter hub from the delivery system … [Read more...] about Getinge’s Atrium Medical has a Class I recall over covered stents
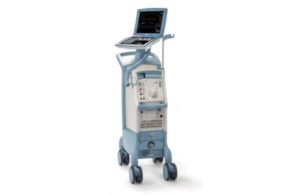
Getinge intra-aortic balloon pump recall is Class I
The FDA determined that Getinge's recall of its Cardiosave Hybrid and CardioSave Rescue devices is Class I, the most serious kind. Datascope/Getinge/Maquet designed the CardioSave Hybrid and CardioSave Rescue intra-aortic balloon pumps (IABP) as cardiac assist devices for patients undergoing cardiac and non-cardiac surgery. They are used in … [Read more...] about Getinge intra-aortic balloon pump recall is Class I