Johnson & Johnson MedTech (NYSE: JNJ) announced that it has a new chief medical & scientific officer for its Electrophysiology unit. Michael Bodner, group chair for Electrophysiology & Neurovascular, posted on LinkedIn that the company appointed Dr. Gregory Michaud to the role. Michaud, a globally recognized electrophysiologist, … [Read more...] about Johnson & Johnson MedTech adds new CMO for its Electrophysiology business
Johnson & Johnson
Johnson & Johnson MedTech launches ultrasound catheter for imaging in cardiac ablation procedures
Johnson & Johnson MedTech (NYSE: JNJ) announced today that it launched the SoundStar Crystal ultrasound catheter. The company launched the catheter in the U.S. for intracardiac echocardiography (ICE) imaging in cardiac ablation procedures. SoundStar Crystal provides clearer, enhanced image quality compared to other ICE catheters, according … [Read more...] about Johnson & Johnson MedTech launches ultrasound catheter for imaging in cardiac ablation procedures
J&J’s Shockwave touts study highlighting the use of IVL-first strategy for women
Johnson & Johnson MedTech‘s Shockwave Medical unit today announced study results highlighting the use of its IVL treatment in female patients. Shockwave announced 30-day primary endpoint results from the EMPOWER CAD study of its intravascular lithotripsy (IVL) therapy. It marks the first prospective, real-world percutaneous … [Read more...] about J&J’s Shockwave touts study highlighting the use of IVL-first strategy for women
Johnson & Johnson MedTech shares strong 3-month Omnypulse data
Johnson & Johnson MedTech announced positive three-month results from a study evaluating its investigational Omnypulse platform. Omnypulse features the Omnypulse catheter and the Trupulse generator. The company offers the catheter as a large-tip, 12 mm device with Carto 3 system integration. It features contact force feedback and enhanced … [Read more...] about Johnson & Johnson MedTech shares strong 3-month Omnypulse data
J&J’s Shockwave enrolls first patient in novel forward IVL study
Johnson & Johnson MedTech‘s Shockwave Medical announced today that it initiated a study evaluating its Javelin coronary IVL catheter. FORWARD CAD, an FDA investigational device exemption (IDE) study, evaluates the safety and efficacy of the company's latest intravascular lithotripsy (IVL) catheter. It aims to treat calcified, … [Read more...] about J&J’s Shockwave enrolls first patient in novel forward IVL study
How Shockwave’s Javelin IVL catheter breaks up blood-blocking calcium where balloons can’t reach
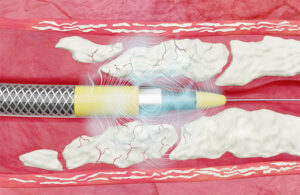
Shockwave’s novel Javelin intravascular lithotripsy catheter focuses acoustic energy forward to clear a path through calcium-clogged blood vessels. Johnson & Johnson MedTech’s Shockwave Medical aims to deliver intravascular lithotripsy (IVL) to a different type of blood vessel with a first-of-its-kind catheter platform. The Javelin … [Read more...] about How Shockwave’s Javelin IVL catheter breaks up blood-blocking calcium where balloons can’t reach
J&J’s Shockwave Medical launches new IVL catheter
Johnson & Johnson MedTech‘s Shockwave Medical today announced the U.S. launch of its Javelin peripheral intravascular lithotripsy (IVL) catheter. The system is designed to modify calcium and cross extremely narrowed vessels in patients with peripheral artery disease (PAD). Shockwave said its novel, non-balloon IVL platform delivers a similar … [Read more...] about J&J’s Shockwave Medical launches new IVL catheter
Johnson & Johnson MedTech resumes Varipulse sales after stroke-related pause
This article originally ran on Feb. 15, 2025. It was updated on Feb. 28 with additional information from the FDA. Johnson & Johnson MedTech announced that it intends to resume the rollout of its Varipulse platform in the U.S. after a temporary pause. Last month, the medtech giant said it temporarily halted all U.S. cases using its … [Read more...] about Johnson & Johnson MedTech resumes Varipulse sales after stroke-related pause
Johnson & Johnson MedTech launches catheter system for stroke
Johnson & Johnson MedTech [WtwhTicker symbol="JNJ"](NYSE: JNJ)[/WtwhTicker] announced today that it launched the CereGlide 92 catheter system for treating acute ischemic stroke. The launch comes just one day after media reports suggested that the company wants to sell its Cerenovus stroke business, the unit that makes the CereGlide … [Read more...] about Johnson & Johnson MedTech launches catheter system for stroke
Johnson & Johnson MedTech wins CE mark for dual-energy catheter
Johnson & Johnson MedTech [WtwhTicker symbol="JNJ"](NYSE: JNJ)[/WtwhTicker] announced today that it received CE mark approval for its dual-energy ThermoCool SmartTouch SF catheter. The irrigated, contact-force sensing catheter features alongside the TruPulse generator. Both offer full integration with the Carto 3 electro-anatomical mapping … [Read more...] about Johnson & Johnson MedTech wins CE mark for dual-energy catheter