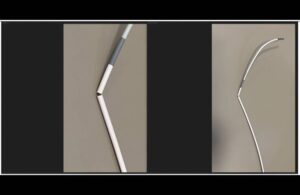
The FDA deemed a recall of catheter guide sheaths from Johnson & Johnson’s (NYSE:JNJ) Cerenovus Class I, the most serious kind. Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between … [Read more...] about FDA says recall of Cerenovus catheter guide sheaths is serious
Neurology
New Synchron CTO Riki Banerjee on BCI manufacturing and outsourcing
After 12 years in Medtronic’s neuromodulation operating unit and two years as R&D VP at Synchron, Riki Banerjee is the brain-computer interface (BCI) developer’s new chief technology officer. More electrodes and thinner electrodes were always goals at Medtronic. But neuro device makers across the industry have faced the difficulties of … [Read more...] about New Synchron CTO Riki Banerjee on BCI manufacturing and outsourcing