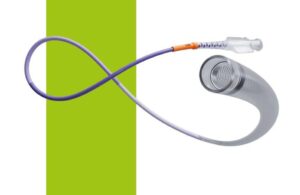
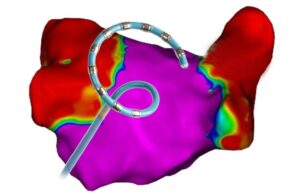
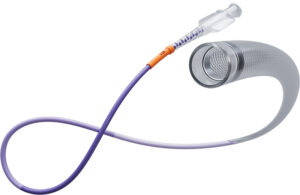
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
johnson & johnson medtech
FDA says recall of Cerenovus catheter guide sheaths is serious
The FDA deemed a recall of catheter guide sheaths from Johnson & Johnson’s (NYSE:JNJ) Cerenovus Class I, the most serious kind. Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between … [Read more...] about FDA says recall of Cerenovus catheter guide sheaths is serious
J&J’s Cerenovus launches next-gen stroke revascularization catheter
Johnson & Johnson MedTech's Cerenovus today announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse. The catheter's indication covers the revascularization of patients suffering from acute ischemic stroke. It joins the company's planned CereGlide family of catheters set to enhance the Cerenovus Stroke … [Read more...] about J&J’s Cerenovus launches next-gen stroke revascularization catheter
Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
Johnson & Johnson MedTech's Biosense Webster today announced Japanese approval for its Varipulse platform for treating AFib. Varipulse treats symptomatic drug-refractory recurrent paroxysmal AFib using pulsed field ablation (PFA). The platform features the Varipulse catheter, a variable-loop multielectrode catheter, the TruPulse generator … [Read more...] about Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
J&J’s Acclarent wins FDA nod for pediatric ear tube balloon dilation system
Johnson & Johnson MedTech unit Acclarent announced that it won a new FDA clearance for its Aera Eustachian tube balloon dilation system. While Acclarent still belongs to Johnson & Johnson, Integra Lifesciences is set to buy it for $275 million next year. While the completion of that deal awaits, the company cleared a regulatory hurdle … [Read more...] about J&J’s Acclarent wins FDA nod for pediatric ear tube balloon dilation system
Cerenovus President Mark Dickinson on the future of stroke care
Cerenovus President Mark Dickinson forecasts the innovative technologies that will advance stroke care in the coming years. It's getting harder to beat aspiration systems for fast and simple thrombectomies to remove blood clots that are blocking oxygen from a stroke patient's brain. That's according to Cerenovus Worldwide President Mark … [Read more...] about Cerenovus President Mark Dickinson on the future of stroke care
J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Johnson & Johnson's Biosense Webster today announced the first completed patient cases in a study of its dual-energy ablation catheter. Biosense Webster designed the ThermoCool SmartTouch SF to deliver both radiofrequency (RF) and pulsed-field ablation (PFA) energy. The SmartPulse pivotal study evaluates the dual-energy system in the … [Read more...] about J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Biosense Webster: catheter ablation leads to lower heart failure risk in AFib patients
Johnson & Johnson's Biosense Webster today announced data supporting the use of catheter ablation in AFib patients. The study, funded by the Irvine, California-based company, looked at heart failure incidence risks in AFib patients. It compared the use of catheter ablation versus antiarrhythmic drugs (AAD). Looking at non-specific catheter … [Read more...] about Biosense Webster: catheter ablation leads to lower heart failure risk in AFib patients
J&J used RWE for expanded indications — and you can, too
Two J&J MedTech leaders shared advice to help medical device developers use real-world evidence (RWE) in FDA submissions. Real-world evidence (RWE) took a big step forward recently when the FDA approved expanded indications for Johnson & Johnson MedTech ablation catheters. For the first time, the federal medical device safety regulator … [Read more...] about J&J used RWE for expanded indications — and you can, too