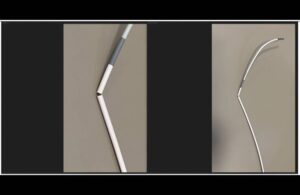
Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between June 14, 2023, and Dec. 14, 2023.
The device, used for precise navigation and access to blood vessels in the brain, helps deliver interventional devices into those vessels. It allows doctors to place devices like stents or coils to treat neurovascular diseases and conditions. The recall occurred because the company received complaints describing fractures of the distal catheter shaft.
Cracks at the far end of the catheter land in an area placed in vessels in the brain where different parts of the catheter join together. Using the affected product may result in a surgical procedural delay, vascular injury or hemorrhage. In extreme, rare occasions, use of the Cerenovus catheter may result in embolism.
The FDA notice lists 3 reported injuries and zero reports of death to date related to the issue.
Johnson & Johnson MedTech, on behalf of Medos, sent all affected customers an Urgent Medical Device Recall. The letter sent by the medtech giant requested that customers examine inventory and quarantine any product subject to the recall. Customers should remove any Cerenovus product subject to the recall and communicate the issue to anyone who needs informing.
Statement on the Cerenovus catheter sheath recall
A spokesperson from Medos International Sàrl issued the following statement regarding the recall:
“With patient safety mind, we issued a voluntary recall of certain lots of our Cerebase DA guide sheath devices. This important action was initiated in response to reports of cracks in the distal shaft of our catheters. We immediately notified the appropriate health agencies as well as affected customers who received detailed instructions on how to identify and return the lots that are subject to the recall.”

