Thermedical announced today that it completed a feasibility study using pulsed field ablation in combination with its SERF ablation system. Weston, Massachusetts–based Thermedical designed its SERF system and Durablate catheter to work with PFA to treat ventricular tachycardia (VT). According to Thermedical, SERF (saline-enhanced radio … [Read more...] about Thermedical completes feasibility study to use SERF with PFA
Pulsed Field Ablation
The top catheter-based innovation news stories of 2024 — so far
Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Biosense Webster reports positive dual-energy ablation results
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
Medtronic has positive data for single-shot mapping ablation catheter
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced positive clinical trial data for its Sphere-360 investigational pulsed field ablation (PFA) catheter technology. The single-shot mapping and ablation catheter uses pulsed field energy to treat patients with paroxysmal AFib. Medtronic presented interim findings on the … [Read more...] about Medtronic has positive data for single-shot mapping ablation catheter
Boston Scientific begins trial of Faraview PFA software with magnetic nav catheter
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it began a new study of its Faraview software module for the Farawave pulsed field ablation (PFA) catheter. The NAVIGATE-PF study evaluates Faraview when used to visualize and track the Farawave Nav PFA catheter for treating AFib. Faraview and the Farawave … [Read more...] about Boston Scientific begins trial of Faraview PFA software with magnetic nav catheter
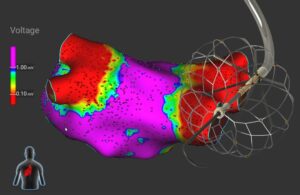
High voltage in the heart: PFA catheter design tips from Biosense Webster
Tushar Sharma at J&J Medtech’s Biosense Webster discusses pulsed-field ablation (PFA) catheter shapes, sensors, electrodes and device design tips.
Biosense Webster’s pulsed-field ablation (PFA) catheter design and development starts in the heart.
That’s where PFA treats atrial fibrillation (AFib) by killing cardiac cells to isolate errant heart signals and prevent them from triggering irregular heartbeats. PFA uses electric fields to permanently open holes in the walls of heart cells … [Read more...] about High voltage in the heart: PFA catheter design tips from Biosense Webster