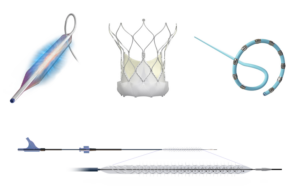
Reflow Medical today announced it received FDA de novo clearance for its Spur Peripheral Retrievable Stent System for infrapopliteal arterial disease. The system is cleared for use in de novo or restenotic lesions following predilatation in below-the-knee (BTK) vessels. It combines a self-expanding stent with an integrated balloon catheter on an … [Read more...] about Reflow Medical wins FDA de novo clearance for Spur stent
Reflow Medical
Reflow Medical expands global reach with European subsidiary
Reflow Medical announced today that it opened a European subsidiary in Landsberg am Lech, Germany, to expand outside the U.S. The company said its strategic expansion strengthens its international presence and enhances its ability to serve markets globally. SVP and GM Knut Saurteig leads the subsidiary, called Reflow Medical Europe GmbH. … [Read more...] about Reflow Medical expands global reach with European subsidiary
Reflow Medical enrolls first patient in coronary sirolimus-eluting retrievable scaffold system study
Reflow Medical announced that investigators enrolled the first patients in a trial of its Spur sirolimus-eluting retrievable scaffold system. DEEPER CORONARY evaluates the Spur Elute stent as a primary treatment for in-stent restenosis (ISR) of the coronary arteries. Spur Elute offers a treatment by transferring a proprietary drug formulation to … [Read more...] about Reflow Medical enrolls first patient in coronary sirolimus-eluting retrievable scaffold system study
The top catheter-based innovation news stories of 2024 — so far
Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Reflow Medical wins CE mark for temporary spur stent
Reflow Medical announced today that it has received a CE mark in the EU for its Bare Temporary Spur Stent System. The system treats de novo or restenotic lesions in the infrapopliteal arteries, followed by a commercially available drug-coated balloon, to enhance drug absorption. The goal is to provide stent-like results while leaving no metal … [Read more...] about Reflow Medical wins CE mark for temporary spur stent
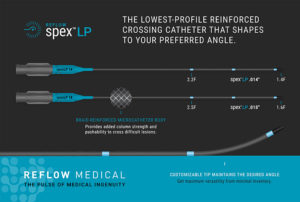
Reflow Medical launches low-profile reinforced support catheters
Reflow Medical today announced it has launched its Reflow Spex Low Profile reinforced support catheters. The Spex LP catheters are designed to provide the lowest profile tip to access and cross the tightest and most complex lesions with a supportive system. They come in 0.014 in. and 0.018 in. sizes and can be combined with the Reflow Spex … [Read more...] about Reflow Medical launches low-profile reinforced support catheters
Reflow Medical lands 510(k) for expanded indication for Wingman catheter
Reflow Medical recently announced that it received FDA clearance for expanded indication of its Wingman Crossing Catheter. The Wingman Catheter is designed to cross peripheral CTOs using an extendable beveled tip that creates a channel to help penetrate or cross the occlusion was a guidewire. Reflow Medical received expanded indication for the … [Read more...] about Reflow Medical lands 510(k) for expanded indication for Wingman catheter