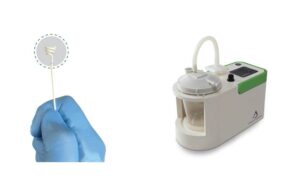
Roivios announced today that it received FDA breakthrough device designation for its JuxtaFlow renal assist device (RAD). The Bahamas-based medical device company designed JuxtaFlow to improve treatments for patients facing kidney disease during cardiac surgery. JuxtaFlow uses a unique, gentle negative pressure technique on the kidneys' … [Read more...] about Roivios wins FDA breakthrough nod for renal assist device
Fresenius Medical Care recalls more than 2 million catheter components
The FDA says a recall of Fresenius Medical Care catheter extension sets and luer lock adapters is Class I, the most serious kind. Fresenius Medical Care initiated the recall of a number of its Stay-Safe luer lock adapters and catheter extensions on Jan. 23, 2024. It extends to nearly 2.2 million devices distributed between March 5, 2003, and … [Read more...] about Fresenius Medical Care recalls more than 2 million catheter components
Thermedical completes feasibility study to use SERF with PFA
Thermedical announced today that it completed a feasibility study using pulsed field ablation in combination with its SERF ablation system. Weston, Massachusetts–based Thermedical designed its SERF system and Durablate catheter to work with PFA to treat ventricular tachycardia (VT). According to Thermedical, SERF (saline-enhanced radio … [Read more...] about Thermedical completes feasibility study to use SERF with PFA
FDA clears computer-assisted thrombectomy system from Penumbra
Penumbra (NYSE:PEN) announced today that it received FDA clearance for and began the launch of its Lightning Flash 2.0 CAVT system. Lightning Flash 2.0 represents the next generation of the company's computer-assisted vacuum thrombectomy (CAVT) technology. It removes the venous thrombus to treat pulmonary emboli (PE). The system features … [Read more...] about FDA clears computer-assisted thrombectomy system from Penumbra
Concept Medical enrolls first patient in drug-coated balloon study
Concept Medical announced today that it commenced the FDA investigational device exemption (IDE) study of its MagicTouch system. MagicTouch, a sirolimus drug-coated balloon (DCB), treats in-stent restenosis (ISR) in coronary artery disease (CAD). Dr. Said Ashraf enrolled the first patient in the MAGICAL-ISR study at the AtlantiCare Institute in … [Read more...] about Concept Medical enrolls first patient in drug-coated balloon study
Cook Medical wins DOD contract for drug-eluting stents
Cook Medical announced today that the U.S. Dept. of Defense (DOD) awarded it an ECAT contract for implantable medical devices. The contract includes implantable vascular medical devices like the Zilver PTX drug-eluting peripheral stent. It also applies to the Zenith aortic endografts and associated interventional devices for treating vascular … [Read more...] about Cook Medical wins DOD contract for drug-eluting stents
Getinge wins European approval for covered stent system
Getinge announced today that it received EU MDR certification for its Advanta V12 covered stent system for patients with aortoiliac occlusive disease (AIOD). The balloon-expandable covered stent's indication aligns with AIOD, which includes treating lesions at the aortic bifurcation. Getinge said EU MDR certification highlights its dedication … [Read more...] about Getinge wins European approval for covered stent system
Nectero Medical closes $96M Series D
Nectero Medical announced today that it closed a Series D financing round worth proceeds of $96 million. Norwest Venture Partners led the round. Boston Scientific, BioStar Capital, Cadence Healthcare Ventures, Aphelion Capital and other firms contributed large investments. Funds add to a $19.5 million Series C — for which Boston Scientific led … [Read more...] about Nectero Medical closes $96M Series D
Biosense Webster reports positive dual-energy ablation results
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
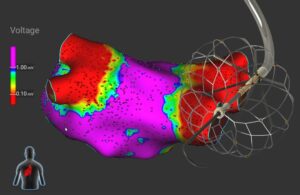
Medtronic has positive data for single-shot mapping ablation catheter
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced positive clinical trial data for its Sphere-360 investigational pulsed field ablation (PFA) catheter technology. The single-shot mapping and ablation catheter uses pulsed field energy to treat patients with paroxysmal AFib. Medtronic presented interim findings on the … [Read more...] about Medtronic has positive data for single-shot mapping ablation catheter