The FDA deemed a recall of catheter guide sheaths from Johnson & Johnson’s (NYSE:JNJ) Cerenovus Class I, the most serious kind. Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between … [Read more...] about FDA says recall of Cerenovus catheter guide sheaths is serious
SafeGuard Surgical wins FDA breakthrough nod for biodegradable stent backed by NFL QB
SafeGuard Surgical announced today that the FDA granted breakthrough device designation to its LeakGuard biodegradable stent. Along with the regulatory milestone, the company announced that it completed its Series A funding round. Tom Pepin, principal at Tapper Ventures, led the financing. NFL quarterback Jameis Winston is also one of the first … [Read more...] about SafeGuard Surgical wins FDA breakthrough nod for biodegradable stent backed by NFL QB
Inari Medical has positive ClotTriever data
Inari Medical (Nasdaq:NARI) announced positive two-year interim results from the CLOUT registry evaluating its ClotTriever system. Principal investigator Dr. David Dexter of Sentara Vascular Specialists in Norfolk, Virginia, presented the data at the American Venous Forum. Results represent the largest prospective, multi-center, two-year … [Read more...] about Inari Medical has positive ClotTriever data
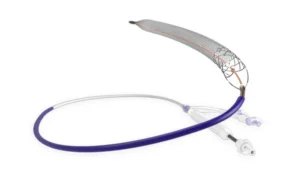
Abbott is launching its latest Navitor TAVI system in the U.S.
Abbott (NYSE:ABT) is launching the latest generation of its Navitor heart valve technology in the U.S., according to one company official. In a post on LinkedIn, Chris Waddell, Abbott’s U.S. VP for transcatheter aortic valve implantation (TAVI), shared the news of the launch. The latest version — called the Navitor Vision — features a range of … [Read more...] about Abbott is launching its latest Navitor TAVI system in the U.S.
Boston Scientific has positive Agent DCB study results
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] reported positive investigational device exemption (IDE) trial data for its Agent drug-coated balloon (DCB). The DCB just this month won FDA approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue … [Read more...] about Boston Scientific has positive Agent DCB study results
Gradient begins pulmonary artery denervation trial in the U.S.
Gradient Denervation Technologies announced today that it won FDA approval to begin an early feasibility study in the U.S. The PreVail-PH2 study evaluates its minimally invasive, ultrasound-based device for treating pulmonary tension with associated heart failure. Paris, France-based Gradient already enrolled the first patient at Duke University … [Read more...] about Gradient begins pulmonary artery denervation trial in the U.S.
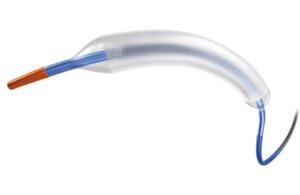
Silk Road Medical launches new transcarotid stent in the U.S.
Silk Road Medical (Nasdaq:SILK) announced today that it launched its tapered EnRoute transcarotid stent system in the U.S. Sunnyvale, California-based Silk Road says this expands upon the prior launch of its EnRoute system by adding additional configurations. It better tailors the transcarotid artery revascularization (TCAR) procedure to the … [Read more...] about Silk Road Medical launches new transcarotid stent in the U.S.
Stereotaxis submits Magic ablation catheter for U.S., European approval
Stereotaxis (NYSE:STXS) announced that it submitted its Magic ablation catheter for both European and U.S. regulatory approval. The company said its FDA and CE mark submissions follow successful clinical results in an ongoing trial. Last month, Stereotaxis announced the first Magic treatments in the European trial supporting its submissions. … [Read more...] about Stereotaxis submits Magic ablation catheter for U.S., European approval
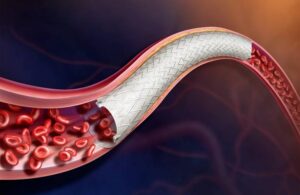
BD initiates IDE trial of stent for treating peripheral arterial disease
BD (NYSE: BDX) announced today that it enrolled the first patient in a trial of its Vascular Covered Stent for treating peripheral arterial disease (PAD). Franklin Lakes, New Jersey–based BD is evaluating the safety and effectiveness of the stent in its AGILITY FDA investigational device exemption (IDE) trial. The self-expanding, low-profile, … [Read more...] about BD initiates IDE trial of stent for treating peripheral arterial disease
FDA approves Boston Scientific’s Agent drug coated balloon
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it received FDA approval for its Agent drug-coated balloon (DCB). The DCB won approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented … [Read more...] about FDA approves Boston Scientific’s Agent drug coated balloon