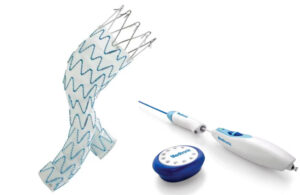
Innova Vascular today announced the successful early commercial use of its Laguna Thrombectomy System in California. The first physicians to use the system — Dr. Raj Khalsa and Dr. John Moriarty — reported positively after their initial cases. Khalsa touted the system's easy preparation, strong trackability in challenging anatomies and … [Read more...] about Innova Vascular has positive early commercial data for thrombectomy system
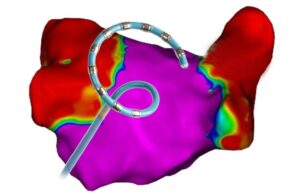
Catheter design was key for the Boston Scientific Farapulse pulsed field ablation system
Boston Scientific Chief Medical Officer Dr. Ken Stein explains how catheter design drives the Farapulse pulsed field ablation system.
Following FDA approval on Jan. 31, Farapulse from Boston Scientific is now the second pulsed field ablation (PFA) system approved to treat atrial fibrillation (AFib) in the U.S.
Medtronic’s PulseSelect PFA System picked up the first FDA approval in December for the treatment of paroxysmal and persistent AFib.
Boston Scientific’s Farapulse, on … [Read more...] about Catheter design was key for the Boston Scientific Farapulse pulsed field ablation system
Medtronic enrolls first patient in aneurysm repair trial
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] announced today that it enrolled the first patient in the Hercules trial comparing abdominal aortic aneurysm repair methods. Hercules compares endosuture aneurysm repair (ESAR) to standard endovascular aneurysm repair (EVAR). It looks at these methods in patients who have an abdominal … [Read more...] about Medtronic enrolls first patient in aneurysm repair trial
Cortex initiates trial of mapping tech for AFib ablation
Cortex announced today that it began its RESOLVE-AF trial evaluating its Ablamap system with the newly release Ablacath mapping technology. Launched by Ajax Health in December 2023, the company already offers the FDA-cleared Ablamap with electrographic flow (EGF). It seeks to pair the system with the newly released Ablacath mapping catheter to … [Read more...] about Cortex initiates trial of mapping tech for AFib ablation
FDA approves Gore low-profile balloon-expandable endoprosthesis
W.L. Gore & Associates announced today that it received FDA approval for a lower-profile Viabahn VBX balloon-expandable endoprosthesis. FDA approval builds on the company's proven VBX stent graft for treating complex vascular disease. The company says it offers the longest balloon-expandable stent on the market (79 mm) and the widest range … [Read more...] about FDA approves Gore low-profile balloon-expandable endoprosthesis
Olympus completes acquisition of GI stent maker Taewoong Medical
Olympus announced today that it closed its acquisition of Taewoong Medical, a Korea-based medical device manufacturer. Taewoong Medical develops gastrointestinal (GI) metallic stents, among other offerings. Olympus announced its intent to acquire the company for $370 million in February 2023. The deal includes $255.5 million upfront, with up to … [Read more...] about Olympus completes acquisition of GI stent maker Taewoong Medical
Surmodics has positive early results from thrombectomy system
Surmodics (Nasdaq:SRDX) today announced the successful early clinical use of its Pounce LP thrombectomy system. Eden Prairie, Minnesota-based Surmodics designed the LP (low-profile) thrombectomy system for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature. It performs this removal in vessels between 3.5 mm … [Read more...] about Surmodics has positive early results from thrombectomy system
Gore enrolls first patients in expandable stent graft trial
W.L. Gore & Associates announced today that it enrolled the first patients in a trial evaluating its Viabahn VBX stent graft. The study compares Gore’s balloon-expandable endoprosthesis to bare metal stenting for patients with complex iliac occlusive disease. It aims to inform practice guidelines around the best modalities suited for … [Read more...] about Gore enrolls first patients in expandable stent graft trial
Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
Johnson & Johnson MedTech's Biosense Webster today announced Japanese approval for its Varipulse platform for treating AFib. Varipulse treats symptomatic drug-refractory recurrent paroxysmal AFib using pulsed field ablation (PFA). The platform features the Varipulse catheter, a variable-loop multielectrode catheter, the TruPulse generator … [Read more...] about Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
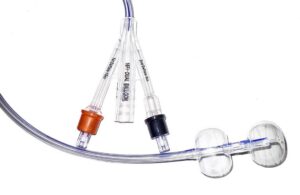
HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical
HR Pharmaceuticals announced that it entered into an exclusive commercial agreement with Poiesis Medical to license catheter technology. The deal centers around Poiesis' dual-balloon catheter, the Duette system. Under the terms, HR Pharmaceuticals has exclusive commercialization rights to Duette in North America. The deal expands HR … [Read more...] about HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical