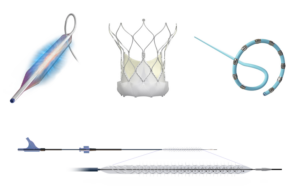
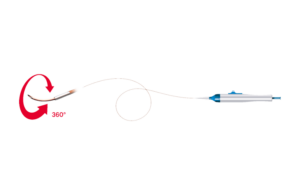
Penumbra (NYSE:PEN) announced today that it received FDA clearance for and began the launch of its Lightning Flash 2.0 CAVT system. Lightning Flash 2.0 represents the next generation of the company's computer-assisted vacuum thrombectomy (CAVT) technology. It removes the venous thrombus to treat pulmonary emboli (PE). The system features … [Read more...] about FDA clears computer-assisted thrombectomy system from Penumbra
Business
Zeus names first chief technology officer
Zeus today announced it named Suresh Sainath as its new chief technology officer, effective immediately. The chief technology officer position is newly created, and Sainath will lead the Orangeburg, S.C.-based polymer extrusion and catheter manufacturing company's efforts to accelerate innovation and leverage the advanced capabilities of its … [Read more...] about Zeus names first chief technology officer
The top catheter-based innovation news stories of 2024 — so far
Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Getinge and Cook Medical today announced they signed a commercial distribution agreement for the iCast covered stent system in the U.S. In this deal, Cook Medical will take over sales, marketing, and distribution rights for the product in the U.S. The iCast covered stent system will continue to be manufactured by Atrium Medical, a Getinge … [Read more...] about Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Cook Medical signs distribution agreement with Bentley for BeBack catheter
Cook Medical today announced it signed a U.S. distribution agreement with Bentley for the BeBack catheter. Under the agreement, Cook Medical will assume commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack catheter to our peripheral intervention portfolio,” Alec Cerchiari, director of product … [Read more...] about Cook Medical signs distribution agreement with Bentley for BeBack catheter
Zeus hires new CEO from Becton Dickinson
Zeus has hired Padraic “Paddy” O’Brien as CEO from Becton, Dickinson and Co. effective April 1, the medical tubing supplier said today. O’Brien is worldwide president of BD Peripheral Intervention, where he “developed an extensive and consistent track record of delivering durable, sustainable growth while creating a winning culture,” Zeus said … [Read more...] about Zeus hires new CEO from Becton Dickinson
Zeus sale to private equity firm EQT closes
Zeus is officially under new ownership after private equity firm EQT closed on its acquisition of the medical tubing supplier. “Today marks the start of a new chapter for our company, and we’re excited to share this update with you,” Zeus said in an email to “industry partners” announcing the deal’s finalization. “We believe this partnership … [Read more...] about Zeus sale to private equity firm EQT closes