Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Abbott
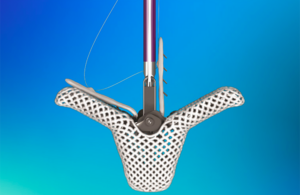
Nitinol grips prevent slips in Abbott’s heart valve clips
Nitinol is a key material in the heart valve clips that Abbott designed for its TriClip and MitraClip transcatheter edge-to-edge repair (TEER) systems. Abbott designed the TriClip system (approved by the FDA in April 2024) for reducing tricuspid valve regurgitation using fourth-generation heart valve clips that Abbott originally developed for … [Read more...] about Nitinol grips prevent slips in Abbott’s heart valve clips
How Abbott created its next-gen RF ablation system
For Abbott, combining two innovations into one AFib-treating system required manufacturing fine-tuning. The project’s R&D director explains. Pulsed field ablation (PFA) has generated a great deal of buzz for its potential to reduce complications in procedures for treating atrial fibrillation (AFib). But even as Abbott is developing its Volt … [Read more...] about How Abbott created its next-gen RF ablation system
Abbott is launching its latest Navitor TAVI system in the U.S.
Abbott (NYSE:ABT) is launching the latest generation of its Navitor heart valve technology in the U.S., according to one company official. In a post on LinkedIn, Chris Waddell, Abbott’s U.S. VP for transcatheter aortic valve implantation (TAVI), shared the news of the launch. The latest version — called the Navitor Vision — features a range of … [Read more...] about Abbott is launching its latest Navitor TAVI system in the U.S.
FDA panel votes in favor of Abbott TriClip TEER system for treating tricuspid regurgitation
Abbott (NYSE:ABT) announced today that an FDA advisory committee ruled that the benefits of its TriClip outweigh the risks in treating tricuspid regurgitation (TR). The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the FDA confirmed this with votes registering 13 to 1 in favor of TriClip’s benefits. Abbott … [Read more...] about FDA panel votes in favor of Abbott TriClip TEER system for treating tricuspid regurgitation
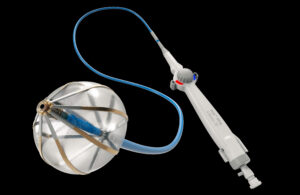
Abbott bets on balloons in pulse field ablation battle
One component stands out as unique when you compare the Abbott Volt against competing pulse field ablation (PFA) systems from Medtronic and Boston Scientific. The Volt cardiac ablation catheter has a balloon, something you won’t see on Medtronic’s PulseSelect PFA catheter — the first of its kind approved by the FDA — or the Farawave catheter in … [Read more...] about Abbott bets on balloons in pulse field ablation battle
The worst catheter-based device recalls of 2023
The U.S. saw seven severe catheter-based device recalls in 2023, according to the FDA's medical device recall database. The agency in 2023 tagged 61 medical device recalls as Class I — the most serious level. For comparison, the FDA recalled 60 devices in 2022, of which four were related to catheter-based devices. Last year, the most serious … [Read more...] about The worst catheter-based device recalls of 2023
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
The biggest cardiovascular stories from TCT 2023
Every year, some of the biggest names in cardiovascular technologies come together in one place for TCT. This year's 35th edition of the Transcatheter Cardiovascular Therapeutics annual scientific symposium was no different in San Francisco. Usual suspects like Medtronic and Abbott had data on display for a range of products, while other big … [Read more...] about The biggest cardiovascular stories from TCT 2023
Abbott has positive data for Esprit drug-eluting resorbable scaffold
Abbott today announced data supporting its Esprit BTK everolimus-eluting resorbable scaffold system. The company designed Esprit BTK to treat people with chronic limb-threatening ischemia (CLTI), a severe stage of peripheral artery disease (PAD). Abbott said its LIFE-BTK trial met both of its primary safety and effectiveness endpoints. It … [Read more...] about Abbott has positive data for Esprit drug-eluting resorbable scaffold