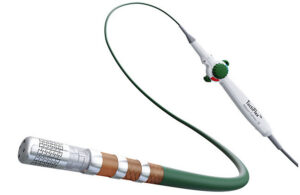
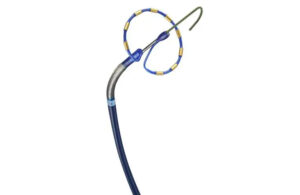
For Abbott, combining two innovations into one AFib-treating system required manufacturing fine-tuning. The project’s R&D director explains. Pulsed field ablation (PFA) has generated a great deal of buzz for its potential to reduce complications in procedures for treating atrial fibrillation (AFib). But even as Abbott is developing its Volt … [Read more...] about How Abbott created its next-gen RF ablation system
Research & Development
Microbot Medical expands U.S. operations to prep for first in-human clinical study
Microbot Medical, the developer of the Liberty endovascular robotic surgical system, is building on the momentum from the positive results of its good laboratory practices (GLP) pre-clinical study. The company is adding a clinical research associate to support its pending investigational device exemption, or IDE, submission to commence its … [Read more...] about Microbot Medical expands U.S. operations to prep for first in-human clinical study
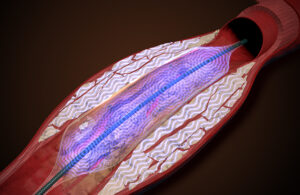
AVS is developing a new method of intravascular lithotripsy
Amplitude Vascular Systems (AVS) is working on a new way to treat severely calcified arterial disease. The name of the company’s peripheral intravascular lithotripsy (IVL) system is Pulse. The Boston-based startup could start enrolling patients in an investigational device exemption (IDE) study as soon as next year. AVS Chief Operating … [Read more...] about AVS is developing a new method of intravascular lithotripsy
Two-armed surgical robot prototype targets pediatric brain tumors
Researchers say they've developed a surgical robot for removing brain tumors in children, and that it could also offer a less invasive, safer option for adult neurosurgery and other procedures. The trick is using hollow, nitinol robot arms to allow neurosurgeons to swap tools during a tumor resection procedure, said Pierre DuPont. He's the chief … [Read more...] about Two-armed surgical robot prototype targets pediatric brain tumors
The top catheter innovation news stories of 2023 — so far
The world of catheter innovation has already seen remarkable strides this year— with some of the most promising medtech delivered into the body via catheter-based procedures. Think of improved ablation for treating AFib, coronary intravascular lithotripsy to combat coronary artery disease, and more. Here, we've highlighted the top five … [Read more...] about The top catheter innovation news stories of 2023 — so far
The biggest cardiology tech stories from ACC.23
Major medtech players presented a range of intriguing studies at a gathering of some of the biggest names in the cardiology tech space. Some of the hottest topics were covered this past week at the American College of Cardiology’s Annual Scientific Session Together With the World Congress of Cardiology (ACC.23/WCC) in New Orleans. Ablation, … [Read more...] about The biggest cardiology tech stories from ACC.23
Biosense Webster marks first cases with dual energy ablation tech
Biosense Webster today announced the first cases with its investigational Thermocool SmartTouch SF dual energy catheter for treating AFib. The system enables doctors performing an ablation to toggle between two types of energy: pulsed-field and radiofrequency. It appears to be the Johnson & Johnson MedTech subsidiary's answer to the dual … [Read more...] about Biosense Webster marks first cases with dual energy ablation tech
Medtronic pulsed-field ablation system exceeds safety goal in study
Medtronic (NYSE:MDT) announced today that its PulseSelect pulsed-field ablation (PFA) system exceeded its safety performance goal in a clinical trial. PulseSelect registered an adverse event rate of 0.7%. Medtronic said that marks one of the lowest adverse event rates of any previous FDA investigational device exemption (IDE) trial for AFib … [Read more...] about Medtronic pulsed-field ablation system exceeds safety goal in study
This surgical robotic arm could 3D bioprint inside the human body
Engineers in Australia say they have developed a miniature robotic arm for 3D printing biomaterial directly on human organs. UNSW Sydney researchers developed the device as a flexible, soft robotic arm for 3D bioprinting. This process fabricates biomedical parts from “bioink” to construct natural, tissue-like structures. Predominantly used for … [Read more...] about This surgical robotic arm could 3D bioprint inside the human body
Alleviant Medical raises $75M for no-implant heart failure treatment
Alleviant Medical has raised $75 million to support a global pivotal trial of its catheter-delivered interatrial shunt to treat heart failure. The FDA provided the Austin, Texas–based company with an Investigational Device Exemption for the clinical trial in November 2022. Researchers have designed the ALLAY-HF study to demonstrate the safety … [Read more...] about Alleviant Medical raises $75M for no-implant heart failure treatment