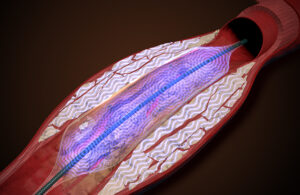
Amplitude Vascular Systems (AVS) is working on a new way to treat severely calcified arterial disease. The name of the company’s peripheral intravascular lithotripsy (IVL) system is Pulse. The Boston-based startup could start enrolling patients in an investigational device exemption (IDE) study as soon as next year. AVS Chief Operating … [Read more...] about AVS is developing a new method of intravascular lithotripsy