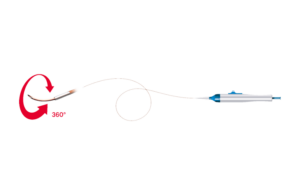
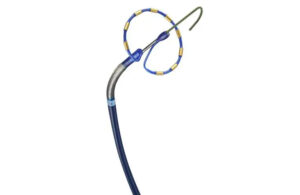
Cook Medical today announced it signed a U.S. distribution agreement with Bentley for the BeBack catheter. Under the agreement, Cook Medical will assume commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack catheter to our peripheral intervention portfolio,” Alec Cerchiari, director of product … [Read more...] about Cook Medical signs distribution agreement with Bentley for BeBack catheter
Nitinol
ProVerum completes enrollment in ProVIDE clinical study for ProVee BPH treatment device
ProVerum this week announced it completed enrollment in its ProVIDE pivotal clinical trial to evaluate the safety and effectiveness of the ProVee system. The ProVIDE clinical trial is a prospective, multicenter, double-blind controlled study that evaluates the safety, performance, and effectiveness of the ProVee system in patients with lower … [Read more...] about ProVerum completes enrollment in ProVIDE clinical study for ProVee BPH treatment device
BD initiates IDE trial of stent for treating peripheral arterial disease
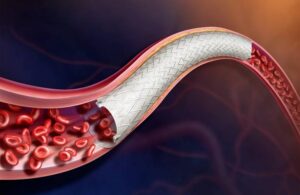
BD (NYSE: BDX) announced today that it enrolled the first patient in a trial of its Vascular Covered Stent for treating peripheral arterial disease (PAD). Franklin Lakes, New Jersey–based BD is evaluating the safety and effectiveness of the stent in its AGILITY FDA investigational device exemption (IDE) trial. The self-expanding, low-profile, … [Read more...] about BD initiates IDE trial of stent for treating peripheral arterial disease
New Synchron CTO Riki Banerjee on BCI manufacturing and outsourcing
After 12 years in Medtronic’s neuromodulation operating unit and two years as R&D VP at Synchron, Riki Banerjee is the brain-computer interface (BCI) developer’s new chief technology officer. More electrodes and thinner electrodes were always goals at Medtronic. But neuro device makers across the industry have faced the difficulties of … [Read more...] about New Synchron CTO Riki Banerjee on BCI manufacturing and outsourcing
Two-armed surgical robot prototype targets pediatric brain tumors
Researchers say they've developed a surgical robot for removing brain tumors in children, and that it could also offer a less invasive, safer option for adult neurosurgery and other procedures. The trick is using hollow, nitinol robot arms to allow neurosurgeons to swap tools during a tumor resection procedure, said Pierre DuPont. He's the chief … [Read more...] about Two-armed surgical robot prototype targets pediatric brain tumors
Medical nitinol processing: How NiTi is turned into wire, tubes and sheets for medical devices
Medical nitinol processing transforms raw nickel-titanium alloy into wire, coils, tubes and sheets for medical and dental device manufacturers. We previously covered nitinol's journey from the earth's crust through high-temperature melting crucibles and forging operations to create nitinol ingots and then smaller, more workable shapes such as … [Read more...] about Medical nitinol processing: How NiTi is turned into wire, tubes and sheets for medical devices
After recall and relaunch, Medtronic wants to go global with its catheter-delivered Harmony valve
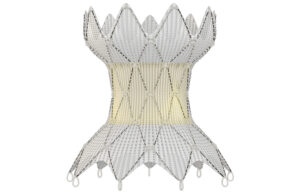
Each Medtronic Harmony valve is sewn by hand to attach laser-cut pig tissue to the nitinol that makes this minimally invasive heart implant possible. Medtronic’s Harmony transcatheter pulmonary valve (TPV) design is paying off after engineers solved a delivery catheter recall and relaunched the system this year. The Harmony TPV uses pig tissue, … [Read more...] about After recall and relaunch, Medtronic wants to go global with its catheter-delivered Harmony valve
How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
Tim Laske, VP of research and business development for Medtronic‘s cardiac ablation solutions business, discusses the challenges of designing devices for the heart and explores the properties of nitinol. The heart is often one of the most underappreciated aspects of human anatomy, and its atrial appendages are often overlooked even in cardiac … [Read more...] about How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
Endosmart partners with Chamfr to provide nitinol components for medical devices
Endosmart today announced it partnered with Chamfr to provide nitinol components for medical devices online. Through the partnership, engineers can order selected nitinol braids, shape-set tubes and wires and grinder wires from Chamfr's website. "Chamfr is giving medical device engineers what they need to quickly drive innovation," Endosmart … [Read more...] about Endosmart partners with Chamfr to provide nitinol components for medical devices
Heraeus Medical Components acquires Pulse Systems
Heraeus Medical Components announced this week that it has acquired Pulse Systems, a precision laser processor and manufacturer of nitinol and other metal-based implants and delivery systems. A key reason for the acquisition is the increasing importance of vascular access and delivery systems to enable new therapies, according to Peter Reimer, … [Read more...] about Heraeus Medical Components acquires Pulse Systems