The Galien Foundation recently announced its nominees of medical device innovations for its 2022 Prix Galien USA awards. There are 24 medical technologies nominated for the annual award this year, up from 18 nominees in 2021. The Galien Foundation’s annual Prix Galien awards highlight devices, biotechnology and pharmaceutical products … [Read more...] about The 24 best medical device innovations of 2022
Plastics
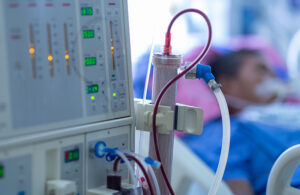
FDA warns of potential toxic risk from Fresenius hemodialysis machines
The FDA is evaluating the risk of exposure to toxic chemicals from silicone tubing used in Fresenius Medical Care (NYSE: FMS) hemodialysis machines. The investigation concerns three models of Fresenius hemodialysis machines: the 2008T, 2008K2, and 2008K. The chemicals — non-dioxin-like (NDL) polychlorinated biphenyl acids (PCBAs) and NDL … [Read more...] about FDA warns of potential toxic risk from Fresenius hemodialysis machines
FDA warns of risk from Medtronic silicone-based EMG endotracheal tubes
The FDA is evaluating the risk of Medtronic (NYSE:MDT) silicone-based electromyogram (EMG) endotracheal tubes following reports of deaths and serious adverse events. The FDA issued the warning last week in a letter to healthcare providers concerning the Medtronic NIM Standard Reinforced EMG Endotracheal Tubes and Medtronic NIM Contact Reinforced … [Read more...] about FDA warns of risk from Medtronic silicone-based EMG endotracheal tubes
Plastics and medical devices: Changes for safety and cost
The move toward polypropylene and polyethylene in medical devices requires new manufacturing and assembly solutions such as ultrasonic and laser welding. Didier Perret, Emerson Plastics are ubiquitous in medical applications thanks to their light weight, durability and flexibility, among other attributes. However, concern has been increasing … [Read more...] about Plastics and medical devices: Changes for safety and cost
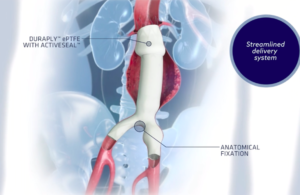
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
Freudenberg Medical opens new HQ in larger Mass. factory
Freudenberg Medical announced today that it has completed construction of a new medical manufacturing operation in Beverly, Mass. The new building will serve as Freudenberg Medical's global headquarters and will manufacture medical devices and components in medical and implant-grade silicones and high-performance plastics. The 36,000 ft2 … [Read more...] about Freudenberg Medical opens new HQ in larger Mass. factory
6 ways the right tubing supplier can improve product development
Partnering with a vertically integrated manufacturer can help engineers optimize designs, improve manufacturability and accelerate new product development. Michael Holt, Integer (Image courtesy of Integer) Medtech design engineers face extraordinary opportunities and challenges created by rapidly advancing clinical markets. To … [Read more...] about 6 ways the right tubing supplier can improve product development
How plasma treatment offers green, cost-efficient preparation of plastics
Surface treatment to prepare medical device components for printing or bonding may require complex cleaning methods or harmful chemicals. Plasma etching for improved adhesion, once limited to low-pressure chambers, is now available at atmospheric pressure. Erhard Krampe, Plasmatreat Conventional methods of surface preparation, treatment … [Read more...] about How plasma treatment offers green, cost-efficient preparation of plastics
How catheters are becoming increasingly complex and efficient
As the neurovascular intervention marketplace accelerates toward miniaturization, innovations such as 2.5:1 peelable heat-shrink tubing (PHST), cut-to-length PHST and multi-filar active catheter solutions will come into their own. Joe Rowan, Junkosha Catheters are becoming increasingly complex in terms of the procedures they can be used … [Read more...] about How catheters are becoming increasingly complex and efficient
Raumedic touts new biocompatible additive
Medical materials company Raumedic says a new biocompatible additive it's developed can further enhance the sliding properties of medical plastic components. The Helmbrechts, Germany–based of medical-grade plastic compounds — which has its U.S. headquarters in Mills River, N.C. — has tested the new additive for in combination with a base polymer … [Read more...] about Raumedic touts new biocompatible additive