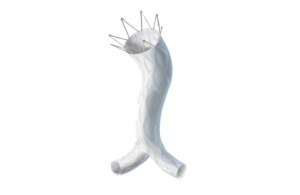
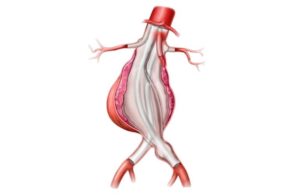
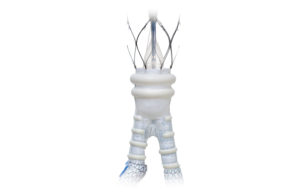
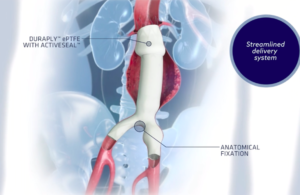
Endologix today announced it received FDA approval for a pre-market approval supplement relating to its AFX2 system. The expanded approval includes an updated warning and the most contemporary clinical information in the labeling for the AFX2 system. Clinical data was added to the instructions for use (IFU) to include: - Final results … [Read more...] about Endologix AFX2 system wins FDA approval for PMA supplement
Endologix
Endologix reports positive results from percutaneous bypass trial
Endologix announced today that 12-month results from a trial of its Detour system demonstrated a low rate of complications. Irvine, California-based Endologix's Detour 2 FDA investigational device exemption (IDE) study was designed to evaluate the safety and effectiveness of the Detour system for percutaneous bypass in the treatment of … [Read more...] about Endologix reports positive results from percutaneous bypass trial
Endologix wins FDA breakthrough nod for aneurysm sealing system
Endologix announced today that the FDA granted breakthrough device designation for its Chimney endovascular aneurysm sealing system (Chevas). Irvine, Calif.–based Endologix designed its Chevas system as an investigational endovascular abdominal aortic aneurysm (AAA) sealing therapy that combines the Nellix 3.5 endograft with parallel visceral … [Read more...] about Endologix wins FDA breakthrough nod for aneurysm sealing system
Endologix launches Alto abdominal stent graft in Canada
Endologix (NSDQ:ELGX) today said it launched its Alto abdominal stent graft in Canada following its recent Health Canada approval. The Alto abdominal stent graft system is a polymer-based therapy for abdominal aortic aneurysm (AAA) patients. It uses a low-profile delivery system with a conformable sealing ring that molds in-situ to the patient's … [Read more...] about Endologix launches Alto abdominal stent graft in Canada
Endologix acquires PQ Bypass
Endologix today announced that it has completed its acquisition of PQ Bypass for an undisclosed amount. PQ Bypass makes the Detour platform that is designed for percutaneous femoral-popliteal bypass, which has been designated as a breakthrough device by the FDA. The Detour system has the company’s Torus stent graft and the PQ crossing device. … [Read more...] about Endologix acquires PQ Bypass
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
The worst catheter-based device recalls of 2020
The U.S. saw nine serious medical device recalls related to catheters in 2020 — up from four the previous year, according to the FDA. The agency 2020 tagged a total of 33 medical device recalls as Class I — the most serious level — down from 49 in 2019. The list of the most serious catheter-based device recalls in 2020 includes products from … [Read more...] about The worst catheter-based device recalls of 2020
Endologix launches abdominal stent graft in Europe
Endologix today announced that it has launched its Alto abdominal stent graft system in Europe. The first implant of the stent graft took place in Stevenage, England, following the company's recent CE mark approval. The device was chosen for that specific patient because of their short, thrombus-lined conical aortic neck. “We are pleased to … [Read more...] about Endologix launches abdominal stent graft in Europe