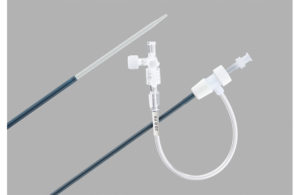
Cook Medical: Flexor Check-Flo introducers and Flexor tuohy-borst side-arm introducers
Cook Medical initiated a recall of its Flexor Check-Flo introducers and Flexor tuohy-borst side-arm introducers on Nov. 24 last year. The FDA designated it a Class I recall on Dec. 28.
The recall involved 37,326 devices, which are used to deliver medical devices to blood vessels. The Flexor devices in the recall have an increased chance of separating at the proximal bond site and could cause serious adverse events, including longer procedure time, a separate procedure to take out a separated piece, blocking blood flow to vital organs, vessel injury and bleeding.
There have been 57 complaints about the device issue and 14 reports of serious injuries, according to an FDA warning on Dec. 23. There have been no reported deaths.
Cook Medical told healthcare providers in an urgent medical device recall notification letter to return the devices and immediately report adverse events to the company’s medical customer relations.
Introducers involved in the recall were made between Feb. 17 and Sept. 29 last year and were distributed between May 23 and Nov. 17.

