Endologix: Ovation iX abdominal stent graft system
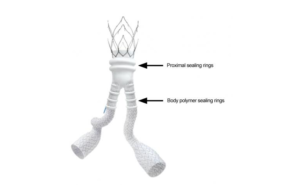
The recall involved 5,403 units, which are intended to treat patients with abdominal aortic or aortoiliac aneurysms. The device uses a polymer-based technology to seal off blood from flowing into the aneurysm and help secure the device in place.
Irvine, Calif.-based Endologix issued the recall due to risks of liquid polymer leaks during implantation. The company said the root cause for most polymer leaks is material weaknesses caused during the manufacturing process. The weakened area may gap or open during use, which can cause the liquid polymer to leak outside of the device as it is filled. When there isn’t enough polymer in the device to seal the aneurysm, blood could continue to flow into the aneurysm and require additional procedures to properly seal it. Liquid polymer could also leak into a patient’s body. The risks include severe allergic-type reactions, unstable blood pressure, tissue damage, organ failure, cardiac arrest, central nervous system problems and death.
Out of 12,763 Ovation iX systems sold between August 2015 and May 2020, there were five deaths — though Endologix clarified that two of the five deaths took place following complications after a polymer leak, according to a June 16 FDA letter.
Endologix replaced the Ovation iX abdominal stent graft system with its FDA-approved Alto abdominal stent graft system. Alto is designed to repair a weakened and bulging section of the aorta below the renal arteries.

