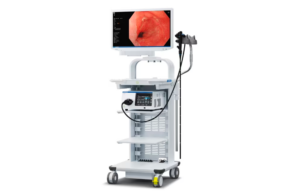
Pentax Medical this week announced it received CE mark approvals for new models of its i20c video endopscope series. The approval covers the Pentax Medical Video Colonoscope EC34-i20c, Pentax Medical Upper GI Scope EG27-i20c and the R/L Knob Adaptor OE-B17. Pentax Medical's i20c Video Endoscope Series, when used with the Inspira Video … [Read more...] about Pentax Medical’s i20c video endoscopes wins CE mark
Endoscopes
FDA clears FujiFilm’s Scale Eye endoscopic imaging tech
FujiFilm today announced it received FDA 510(k) clearance for its Scale Eye endoscopic imaging technology. Scale Eye is part of the company's expanding Eluxeo endoscopic imaging system. It has a laser-equipped colonoscope and endoscopy support software that displays linear or circular vital measurements or scales on an endoscopy monitor. The … [Read more...] about FDA clears FujiFilm’s Scale Eye endoscopic imaging tech
Olympus bronchoscopes in Class I recall could lead to burns, fire
The FDA labeled a recall of Olympus bronchoscopes that could lead to burns or fire Class I, the most serious kind. Olympus reports 192 complaints related to the issue, including four injuries. The company has received no reports of death. This recall — a correction, not a product removal — applies to Olympus bronchofiberscopes and … [Read more...] about Olympus bronchoscopes in Class I recall could lead to burns, fire
Olympus has more warnings over bronchoscopes
Olympus has initiated a voluntary field corrective action following reports of endobronchial combustion incidents involving bronchoscopes. The field corrective action — announced Nov. 9 — is taking place after incidents occurred during medical procedures utilizing high-frequency therapy equipment in environments rich in oxygen, according to the … [Read more...] about Olympus has more warnings over bronchoscopes
Olympus launches Evis X1 endoscopy system
Olympus recently announced it launched the Evis X1 endoscopy system in the U.S. The Evis X1 received FDA clearance earlier this year with two compatible endoscopes, the GIF-1100 gastrointestinal videoscope and the CF-HQ1100DL/I colonovideoscope. It is indicated for use in the upper digestive tract and the lower digestive tract. Evis X1 has an … [Read more...] about Olympus launches Evis X1 endoscopy system
Olympus corrective action covers laser-compatible bronchoscopes
Olympus is warning health providers about incompatible laser use with bronchoscopes after receiving reports of endobronchial combustion, serious patient injury, and one death. The Japanese medtech giant said on July 3 that its voluntary field corrective action involves 32 BF series endoscope models, 19 of which it distributes in the U.S. The … [Read more...] about Olympus corrective action covers laser-compatible bronchoscopes
Olympus to establish series of digital centers based on Odin Vision’s cloud-AI tech
Olympus announced that it plans to establish a series of digital excellence centers (DECs) following its acquisition of Odin Vision. London-based Odin Vision develops a portfolio of commercially available computer-aided detection/diagnostic (CAD) solutions. It also holds an innovation pipeline of cloud-enabled applications. Olympus agreed to … [Read more...] about Olympus to establish series of digital centers based on Odin Vision’s cloud-AI tech
FDA clears Olympus endoscopy systems and endoscopes
Olympus today announced it received FDA clearance for its new Evis X1 endoscopy system and two compatible gastrointestinal endoscopes. The FDA clearance covers Olympus’ GIF-1100 gastrointestinal videoscope indicated for use in the upper digestive tract, and the CF-HQ1100DL/I colonovideoscope indicated for use in the lower digestive … [Read more...] about FDA clears Olympus endoscopy systems and endoscopes
EvoEndo CEO to leave, replacement named
EvoEndo announced today announced a new CEO and COO along with the departure of previous CEO Heather Underwood. Denver-based EvoEndo, which develops transnasal endoscopy technology, named Jonathan Hartmann as president and CEO. It appointed Ryan Hartman to the COO position. Underwood intends to remain with the company through April to support … [Read more...] about EvoEndo CEO to leave, replacement named
Cook Medical, Vizient ink contract for endoscopy devices
Cook Medical announced today that it received a contract with Vizient covering the company's endoscopy devices. The contract enables Cook to continue offering its endoscopy devices at negotiated pricing terms to Vizient members. “We are honored to have been chosen as an Endoscopy contracted supplier with Vizient," said Rick Simms, Cook … [Read more...] about Cook Medical, Vizient ink contract for endoscopy devices