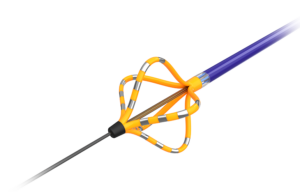
Roivios announced today that it received FDA breakthrough device designation for its JuxtaFlow renal assist device (RAD). The Bahamas-based medical device company designed JuxtaFlow to improve treatments for patients facing kidney disease during cardiac surgery. JuxtaFlow uses a unique, gentle negative pressure technique on the kidneys' … [Read more...] about Roivios wins FDA breakthrough nod for renal assist device
Catheters
Fresenius Medical Care recalls more than 2 million catheter components
The FDA says a recall of Fresenius Medical Care catheter extension sets and luer lock adapters is Class I, the most serious kind. Fresenius Medical Care initiated the recall of a number of its Stay-Safe luer lock adapters and catheter extensions on Jan. 23, 2024. It extends to nearly 2.2 million devices distributed between March 5, 2003, and … [Read more...] about Fresenius Medical Care recalls more than 2 million catheter components
Thermedical completes feasibility study to use SERF with PFA
Thermedical announced today that it completed a feasibility study using pulsed field ablation in combination with its SERF ablation system. Weston, Massachusetts–based Thermedical designed its SERF system and Durablate catheter to work with PFA to treat ventricular tachycardia (VT). According to Thermedical, SERF (saline-enhanced radio … [Read more...] about Thermedical completes feasibility study to use SERF with PFA
FDA clears computer-assisted thrombectomy system from Penumbra
Penumbra (NYSE:PEN) announced today that it received FDA clearance for and began the launch of its Lightning Flash 2.0 CAVT system. Lightning Flash 2.0 represents the next generation of the company's computer-assisted vacuum thrombectomy (CAVT) technology. It removes the venous thrombus to treat pulmonary emboli (PE). The system features … [Read more...] about FDA clears computer-assisted thrombectomy system from Penumbra
The top catheter-based innovation news stories of 2024 — so far
Catheter innovations have had a remarkable year so far, with many receiving regulatory approval as they prepare to enter the market. The pulsed field ablation market heated up in 2023 and continues to make waves this year with more companies entering the space. Intravascular lithotripsy is having its moment as well – Shockwave Medical was … [Read more...] about The top catheter-based innovation news stories of 2024 — so far
Nectero Medical closes $96M Series D
Nectero Medical announced today that it closed a Series D financing round worth proceeds of $96 million. Norwest Venture Partners led the round. Boston Scientific, BioStar Capital, Cadence Healthcare Ventures, Aphelion Capital and other firms contributed large investments. Funds add to a $19.5 million Series C — for which Boston Scientific led … [Read more...] about Nectero Medical closes $96M Series D
Biosense Webster reports positive dual-energy ablation results
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
Medtronic has positive data for single-shot mapping ablation catheter
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced positive clinical trial data for its Sphere-360 investigational pulsed field ablation (PFA) catheter technology. The single-shot mapping and ablation catheter uses pulsed field energy to treat patients with paroxysmal AFib. Medtronic presented interim findings on the … [Read more...] about Medtronic has positive data for single-shot mapping ablation catheter
Boston Scientific begins trial of Faraview PFA software with magnetic nav catheter
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it began a new study of its Faraview software module for the Farawave pulsed field ablation (PFA) catheter. The NAVIGATE-PF study evaluates Faraview when used to visualize and track the Farawave Nav PFA catheter for treating AFib. Faraview and the Farawave … [Read more...] about Boston Scientific begins trial of Faraview PFA software with magnetic nav catheter
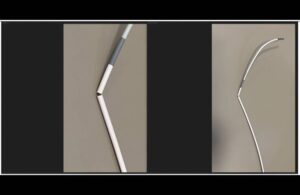
FDA says recall of Cerenovus catheter guide sheaths is serious
The FDA deemed a recall of catheter guide sheaths from Johnson & Johnson’s (NYSE:JNJ) Cerenovus Class I, the most serious kind. Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between … [Read more...] about FDA says recall of Cerenovus catheter guide sheaths is serious