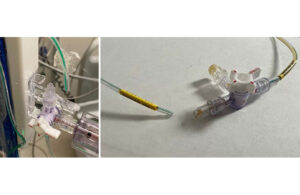
The FDA today designated a Medtronic recall of Duet external drainage and monitoring system catheter tubing as Class I, its most serious level. Medtronic Neurosurgery initiated the recall on Jan. 22. According to the FDA, it involves 45,176 devices distributed from May 3, 2021, to Jan. 9, 2024. The model numbers involved are 46913, 46914, 46915, … [Read more...] about Medtronic has a serious catheter tubing recall
Catheters
Zeus sale to private equity firm EQT closes
Zeus is officially under new ownership after private equity firm EQT closed on its acquisition of the medical tubing supplier. “Today marks the start of a new chapter for our company, and we’re excited to share this update with you,” Zeus said in an email to “industry partners” announcing the deal’s finalization. “We believe this partnership … [Read more...] about Zeus sale to private equity firm EQT closes
Gradient begins pulmonary artery denervation trial in the U.S.
Gradient Denervation Technologies announced today that it won FDA approval to begin an early feasibility study in the U.S. The PreVail-PH2 study evaluates its minimally invasive, ultrasound-based device for treating pulmonary tension with associated heart failure. Paris, France-based Gradient already enrolled the first patient at Duke University … [Read more...] about Gradient begins pulmonary artery denervation trial in the U.S.
Stereotaxis submits Magic ablation catheter for U.S., European approval
Stereotaxis (NYSE:STXS) announced that it submitted its Magic ablation catheter for both European and U.S. regulatory approval. The company said its FDA and CE mark submissions follow successful clinical results in an ongoing trial. Last month, Stereotaxis announced the first Magic treatments in the European trial supporting its submissions. … [Read more...] about Stereotaxis submits Magic ablation catheter for U.S., European approval
FDA approves Boston Scientific’s Agent drug coated balloon
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] announced today that it received FDA approval for its Agent drug-coated balloon (DCB). The DCB won approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented … [Read more...] about FDA approves Boston Scientific’s Agent drug coated balloon
Boston Scientific warns on fragment embolization in some PolarSheath devices
Boston Scientific has issued an urgent field safety notice for some of its PolarSheath steerable sheaths due to the risk of embolization. The company is removing specific batches of PolarSheath steerable sheaths due to a tooling error in manufacturing, which may have caused delimitation of the inner lumen of the sheath shaft in a subset of … [Read more...] about Boston Scientific warns on fragment embolization in some PolarSheath devices
Ablation tech developer Adagio Medical to go public in SPAC deal
Arya Sciences Acquisition Corp IV agreed to a business combination with Adagio Medical, taking the cardiac ablation company public. Upon the closing of the transaction, Adagio is set to become a subsidiary of Aja Holdco, still operating under its existing management team and under the name "Adagio Medical." Arya expects to list the combined … [Read more...] about Ablation tech developer Adagio Medical to go public in SPAC deal
FDA panel votes in favor of Abbott TriClip TEER system for treating tricuspid regurgitation
Abbott (NYSE:ABT) announced today that an FDA advisory committee ruled that the benefits of its TriClip outweigh the risks in treating tricuspid regurgitation (TR). The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the FDA confirmed this with votes registering 13 to 1 in favor of TriClip’s benefits. Abbott … [Read more...] about FDA panel votes in favor of Abbott TriClip TEER system for treating tricuspid regurgitation
Freudenberg Medical establishes second production facility in Costa Rica
NEWS RELEASE: Freudenberg Medical establishes second production facility in Costa Rica $25 million investment to meet growing global demand for medical devices and precision components Expansion to quadruple manufacturing footprint for high volume minimally invasive catheters Creation of 600 new jobs, fostering growth for local … [Read more...] about Freudenberg Medical establishes second production facility in Costa Rica
J&J’s Cerenovus launches next-gen stroke revascularization catheter
Johnson & Johnson MedTech's Cerenovus today announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse. The catheter's indication covers the revascularization of patients suffering from acute ischemic stroke. It joins the company's planned CereGlide family of catheters set to enhance the Cerenovus Stroke … [Read more...] about J&J’s Cerenovus launches next-gen stroke revascularization catheter