The company is removing specific batches of PolarSheath steerable sheaths due to a tooling error in manufacturing, which may have caused delimitation of the inner lumen of the sheath shaft in a subset of PolarSheath devices. The error could result in the embolization of a fragment during use, such as flushing the sheath or introducing the dilator or ablation catheter.
Liner fragment dislodgment could result in a stroke, according to the company. Other potential harms include myocardial ischemia or another distal vascular occlusion.
There have been no complaints or patient harm reported from this issue, according to the urgent field safety notice.
More about the removal
Boston Scientific issued a warning for PolarSheath devices manufactured between Nov. 21, 2023 and Jan. 31, 2024, distributed in Europe and Japan. Devices affected are listed below:

The company recommends that further distribution or use of the affected products cease immediately and to fill out the verification form sent to affected parties.
What is the PolarSheath steerable sheath?
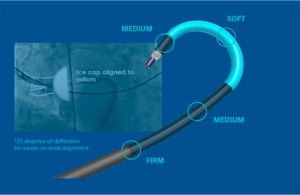
PolarSheath is a single-use, steerable percutaneous introducer sheath that Boston Scientific designed for maneuverability of the company’s PolarX cryoablation system catheter, which is advanced through the sheath and into cardiac chambers.
It has a long, gradual taper from dilator to sheath, providing atraumatic entry at the femoral site and transeptal crossing location. A greater distal sheath deflection increases alignment capabilities for easier balloon positioning.

