Medtronic Neurosurgery initiated the recall on Jan. 22. According to the FDA, it involves 45,176 devices distributed from May 3, 2021, to Jan. 9, 2024. The model numbers involved are 46913, 46914, 46915, 46916, and 46917.
The Duet external drainage and monitoring system provides temporary drainage of cerebrospinal fluid (CSF) or CSF sampling in people who have surgery for descending thoracic aortic aneurysm or descending thoraco-abdominal aortic aneurysm. It’s also for people who have had these types of surgeries and develop symptoms like paraplegia.
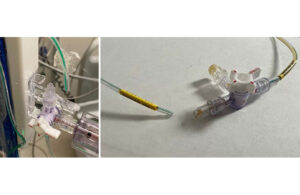
Medtronic is recalling the Duet EDMS catheter tubing due to a potential for catheter disconnection from the patient line stopcock connectors. According to the FDA notice, disconnection could result in infections, cerebrospinal fluid leakage, overdrainage of cerebrospinal fluid, and abnormality of the ventricles. The agency added that uncontrolled overdrainage of cerebral spinal fluid could lead to neurological injury or death if the disconnection is undetected.
The FDA says there have been 26 reports of injuries related to the recall, but no reports of death.
Medtronic is requesting health provider customers to identify and quarantine any unused impacted products. They should check all components for damage and that all connections are secure and leak-free. Patients on a Duet EDMS with a leak or disconnection should be changed to a new alternative device utilizing a sterile technique. Medtronic recommended not changing the device if the Duet EDMS is working as intended.

