The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the FDA confirmed this with votes registering 13 to 1 in favor of TriClip’s benefits. Abbott submitted TriClip, a transcatheter edge-to-edge repair (TEER), to the FDA for premarket approval in March 2023. The company expects a decision on that submission from the FDA this year. The FDA will consider the panel’s vote when making its final decision.
If approved — which could prove more likely with the panel’s affirmative vote — it would take on the recently approved Edwards Evoque tricuspid valve, which became the first transcatheter therapy approved to treat TR earlier this month. While they both treat TR, Abbott labeled TriClip a first-of-its-kind minimally invasive device. It specifically designed TriClip to treat the difficult-to-access tricuspid valve.
Analysts previously said they expect approval for TriClip, which received its most recent CE mark in 2021. It holds approval in more than 50 countries.
“Tricuspid regurgitation can put added strain on the heart and lead to other cardiovascular issues, which can significantly worsen a person’s quality of life, but historically there have been few treatment options,” said Dr. Lars Søndergaard, chief medical officer and divisional VP of medical affairs of Abbott’s structural heart business. “Abbott recognized the unmet need for people with this condition and explored the use of our proven clip-based technology to find a truly life-changing intervention. TriClip offers an urgently needed alternative that is safe and effective for people who require tricuspid valve repair but are not able to withstand surgery.”
More about the Abbott TriClip system and the FDA panel’s decision
TR occurs when the tricuspid valve fails to close properly, causing a leak that allows blood to flow backward in the heart. TriClip could offer a new option for those who continue to have symptoms or persistent TR despite treatment with medical therapy.
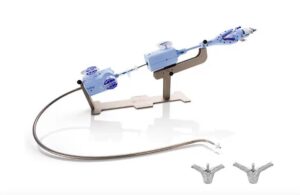
Delivered through a vein in the leg, the TriClip TEER technology clips together a portion of leaflets. It repairs the tricuspid valve and helps blood flow in the right direction without the need for open-heart surgery.
The panel based its decision on clinical data from the TRILUMINATE pivotal trial and expert testimony. Abbott most recently reported positive results from that trial at TCT in October. Results included significant improvements in quality of life along with an excellent safety profile.
After reviewing TRILUMINATE data and listening to testimony, the panel voted on safety, effectiveness and risk/benefit profile. On the question of the existence of enough data supporting safety, the panel voted 14 to 0 in favor. For reasonable assurance of effectiveness, the panel voted 12 to 2 in favor. On the final question of benefit versus risk, the vote came in at 13 to 1 in favor.
“Tricuspid regurgitation can cause fatigue, shortness of breath, irregular heart rhythms, swelling and organ dysfunction. As the disease progresses, it is often debilitating and impacts a person’s ability to live the life they want,” said David Adams, M.D., chairman of the Dept. of Cardiovascular Surgery at the Icahn School of Medicine at Mount Sinai and cardiac surgeon-in-chief of the Mount Sinai Health System, who served as co-principal investigator of the TRILUMINATE pivotal trial. “In the TRILUMINATE pivotal trial, we found that 90% of people who received tricuspid valve repair with the TriClip implant experienced a marked improvement in the severity of their TR with unprecedented procedural safety, and their improvement in quality of life was sustained at one year.
“The TriClip system ushers in a new era of structural therapy for patients suffering from severe tricuspid valve disease.”
The analysts’ view
BTIG analysts Marie Thibault and Sam Eiber say the favorable vote increased their confidence in FDA approval “in the coming months.” They noted that it took between two and three months after the meeting for the last two products reviewed by the same panel to receive approval. Those were the renal denervation products from Recor Medical and Medtronic. Recor’s received a favorable vote, while Medtronic didn’t, but both systems received FDA approval in the end.
“Management has become increasingly bullish on the TR opportunity, despite being a smaller patient population than mitral regurgitation (MR), given there are limited treatment options for patients with TR,” the analysts note.
The analysts say the lone holdout on the risk/benefit vote wanted more information or a better definition on which patients benefit most. He indicated that he may have voted yes if the proposed instructions for use were more narrow in scope.
Panelists cited early and sustained improvements out to 12 months for TriClip as a plus, the analysts said. Most agreed that Abbott could do more work on identifying which patients would benefit most. They also pointed to how to account for potential future right heart interventions and the importance of a trained imaging team.

