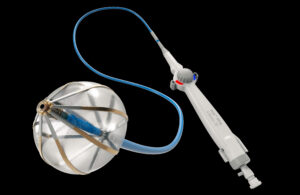
Innova Vascular today announced the successful early commercial use of its Laguna Thrombectomy System in California. The first physicians to use the system — Dr. Raj Khalsa and Dr. John Moriarty — reported positively after their initial cases. Khalsa touted the system's easy preparation, strong trackability in challenging anatomies and … [Read more...] about Innova Vascular has positive early commercial data for thrombectomy system
Catheters
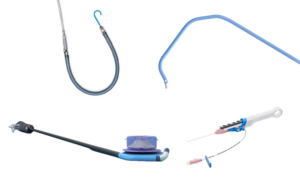
Catheter design was key for the Boston Scientific Farapulse pulsed field ablation system
Boston Scientific Chief Medical Officer Dr. Ken Stein explains how catheter design drives the Farapulse pulsed field ablation system.
Following FDA approval on Jan. 31, Farapulse from Boston Scientific is now the second pulsed field ablation (PFA) system approved to treat atrial fibrillation (AFib) in the U.S.
Medtronic’s PulseSelect PFA System picked up the first FDA approval in December for the treatment of paroxysmal and persistent AFib.
Boston Scientific’s Farapulse, on … [Read more...] about Catheter design was key for the Boston Scientific Farapulse pulsed field ablation system
Cortex initiates trial of mapping tech for AFib ablation
Cortex announced today that it began its RESOLVE-AF trial evaluating its Ablamap system with the newly release Ablacath mapping technology. Launched by Ajax Health in December 2023, the company already offers the FDA-cleared Ablamap with electrographic flow (EGF). It seeks to pair the system with the newly released Ablacath mapping catheter to … [Read more...] about Cortex initiates trial of mapping tech for AFib ablation
Vena Medical achieves first-in-patient for its MicroAngioscope
Vena Medical this week announced that physicians at The Ottawa Hospital (TOH) were the first to use its stroke technology, the MicroAngioscope. Dr. Robert Fahed, an interventional neuroradiologist at TOH, used the MicroAngioscope to treat a patient who had repeated strokes. According to TOH, the device's advanced and high-resolution imaging … [Read more...] about Vena Medical achieves first-in-patient for its MicroAngioscope
FDA clears AngioDynamics’ Auryon XL catheter
AngioDynamics announced today that it received FDA 510(k) clearance for its Auryon XL catheter for use with the Auryon atherectomy system in treating peripheral arterial disease (PAD). The Auryon XP catheter is a 225 cm radial access catheter available in 0.9 mm and 1.5 mm diameters to expand treatment access points in atherectomy procedures in … [Read more...] about FDA clears AngioDynamics’ Auryon XL catheter
Abbott bets on balloons in pulse field ablation battle
One component stands out as unique when you compare the Abbott Volt against competing pulse field ablation (PFA) systems from Medtronic and Boston Scientific. The Volt cardiac ablation catheter has a balloon, something you won’t see on Medtronic’s PulseSelect PFA catheter — the first of its kind approved by the FDA — or the Farawave catheter in … [Read more...] about Abbott bets on balloons in pulse field ablation battle
Surmodics has positive early results from thrombectomy system
Surmodics (Nasdaq:SRDX) today announced the successful early clinical use of its Pounce LP thrombectomy system. Eden Prairie, Minnesota-based Surmodics designed the LP (low-profile) thrombectomy system for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature. It performs this removal in vessels between 3.5 mm … [Read more...] about Surmodics has positive early results from thrombectomy system
The worst catheter-based device recalls of 2023
The U.S. saw seven severe catheter-based device recalls in 2023, according to the FDA's medical device recall database. The agency in 2023 tagged 61 medical device recalls as Class I — the most serious level. For comparison, the FDA recalled 60 devices in 2022, of which four were related to catheter-based devices. Last year, the most serious … [Read more...] about The worst catheter-based device recalls of 2023
FastWave Medical kicks off first-in-human study of its IVL tech
FastWave Medical announced the successful completion of enrollment for its first-in-human study of its peripheral intravascular lithotripsy (IVL) system to treat calcified cardiovascular disease. Dr. Miguel Montero-Baker of Houston Methodist Hospital and the Hope Vascular & Podiatry Clinic and Dr. Venkatesh Ramaiah of HonorHealth Vascular … [Read more...] about FastWave Medical kicks off first-in-human study of its IVL tech
Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Cook Medical recently announced that its Slip-Cath Beacon Tip hydrophilic selective catheter is once again available in the United States and Canada. This reintroduction follows a collaborative effort with medical professionals to refine the product to meet clinical needs better. The Slip-Cath is designed for vascular and non-vascular … [Read more...] about Cook Medical brings Slip-Cath Beacon Tip back to the U.S.