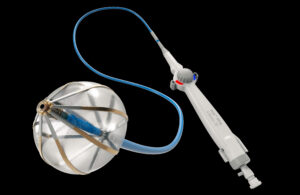
The Volt cardiac ablation catheter has a balloon, something you won’t see on Medtronic’s PulseSelect PFA catheter — the first of its kind approved by the FDA — or the Farawave catheter in the Farapulse PFA system that Boston Scientific expects will soon win approval.
The Abbott Volt’s balloon-in-basket is designed to support the efficient deployment of energy into the tissue during cardiac ablation to treat atrial fibrillation (AFib). That energy creates lesions, scarring the heart tissue to block the irregular electrical signals that disrupt the heart’s normal rhythm.
Abbott said the catheter’s design improves the accuracy, quality and efficiency of ablation to minimize the number of applications needed to treat a patient, as well as potential side effects.
“We have data showing if you use a balloon versus a basket without the balloon it makes a difference [in] lesion depths by 20% to 30%,” said Dr. Christopher Piorkowski, the chief medical officer of Abbott’s electrophysiology division, in an interview with Medical Design & Outsourcing.
To further maximize efficient energy transfer into the heart tissue, Abbott designed the device with electrodes that only face outward and splines that are flat, not round.
Based on Abbott’s bench and animal testing data, the Volt design can drive chronic lesion depths of 6 to 8 mm, Piorkowski said, calling those depths “very sufficient for the left atrium and pulmonary vein isolation.”
At the same time, the balloon acts as an insulator for the blood inside the patient’s beating heart, reducing thermal effects such as bubble formation. Piorkowski said the Volt also uses less voltage for ablation than competing PFA devices, reducing the risk of thermal effects. (He declined to say how much voltage Abbott’s system uses or to quantify the difference.)
The balloon helps stabilize the basket inside the pulmonary vein, where the device’s round shape allows a physician to place the catheter at the ablation target site, apply energy, and rotate the device slightly to achieve a spline offset for a second energy application. In tests, physicians are using three to four applications per treatment.
“That is way below everything that is currently being done with PFA in the clinical field,” he said.

