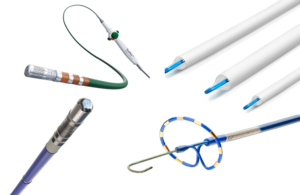
Surmodics (Nasdaq:SRDX) announced today that it commercially launched the Pounce XL thrombectomy system for clot removal. Pounce XL joins the larger suite of Pounce thrombectomy systems. They offer rapid endovascular removal of acute or chronic clot from peripheral arteries. Eden Prairie, Minnesota-based Surmodics designed Pounce to enable … [Read more...] about Surmodics launches Pounce XL thrombectomy system
surmodics
FTC challenges private equity firm’s acquisition of Surmodics
The U.S. Federal Trade Commission (FTC) has sued to block the planned acquisition of medical device company Surmodics by GTCR. According to a news release, GTCR also owns a majority stake in Biocoat. The FTC says Biocoat is the second-largest provider of outsourced hydrophilic medical device coatings, behind only Surmodics. As a result, the FTC … [Read more...] about FTC challenges private equity firm’s acquisition of Surmodics
Surmodics reports first clinical use of thrombectomy system
Surmodics (Nasdaq:SRDX) today announced the successful early clinical use of its Pounce XL thrombectomy system. The early use comes as part of a limited market release. Surmodics plans for a full commercial launch planned for after the initial rollout. Pounce XL received FDA 510(k) clearance in September 2024. Dr. Walter Rizzoni used the system … [Read more...] about Surmodics reports first clinical use of thrombectomy system
FDA clears Surmodics’ Pounce XL thrombectomy system
Surmodics (Nasdaq:SRDX) announced today that it received FDA 510(k) clearance for its Pounce XL thrombectomy system. The FDA indicated Pounce XL for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature in vessels 5.5–10 mm in diameter. That makes it suitable for iliac, femoral, and other arteries within this … [Read more...] about FDA clears Surmodics’ Pounce XL thrombectomy system
Surmodics to be acquired by private equity firm for $627M
Surmodics (Nasdaq:SRDX) announced today that it agreed to be acquired by GTCR, a private equity firm with healthcare interests. Eden Prairie, Minnesota-based Surmodics develops a range of technologies, including drug-coated balloons and thrombectomy systems. Its SurVeil DCB received a long-awaited FDA approval in June 2023. The company also … [Read more...] about Surmodics to be acquired by private equity firm for $627M
Surmodics has positive early results from thrombectomy system
Surmodics (Nasdaq:SRDX) today announced the successful early clinical use of its Pounce LP thrombectomy system. Eden Prairie, Minnesota-based Surmodics designed the LP (low-profile) thrombectomy system for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature. It performs this removal in vessels between 3.5 mm … [Read more...] about Surmodics has positive early results from thrombectomy system
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
Surmodics has positive data on multiple drug-coated balloons
Surmodics (Nasdaq:SRDX) today announced positive study results for two of its drug-coated balloon (DCB) technologies. The company presented data supporting the SurVeil and Sundance DCBs at the 50th Annual VEITH Symposium in New York. In the TRANSCEND trial, Surmodics evaluated the safety and efficacy of SurVeil versus the Medtronic In.Pact … [Read more...] about Surmodics has positive data on multiple drug-coated balloons
Surmodics launches new medical device coating technology
Surmodics (Nasdaq:SRDX) announced today that it launched its most advanced hydrophilic medical device coating technology. Eden Prairie, Minnesota-based Surmodics designed the Preside coatings to complement its existing Serene hydrophilic coatings. Preside offers a unique, low-friction and low-particulate generation coating. It further enhances … [Read more...] about Surmodics launches new medical device coating technology
Surmodics more than doubles sales in Q3
Surmodics (Nasdaq:SRDX) this week announced third-quarter results that beat the overall consensus on Wall Street. The Eden Prairie, Minnesota-based company reported profits of $7.3 million, or 52¢ per share, on sales of $52.5 million for the three months ended June 30. Compared to Q3 2022, the company had a sales gain of 111.2% and profit gains … [Read more...] about Surmodics more than doubles sales in Q3