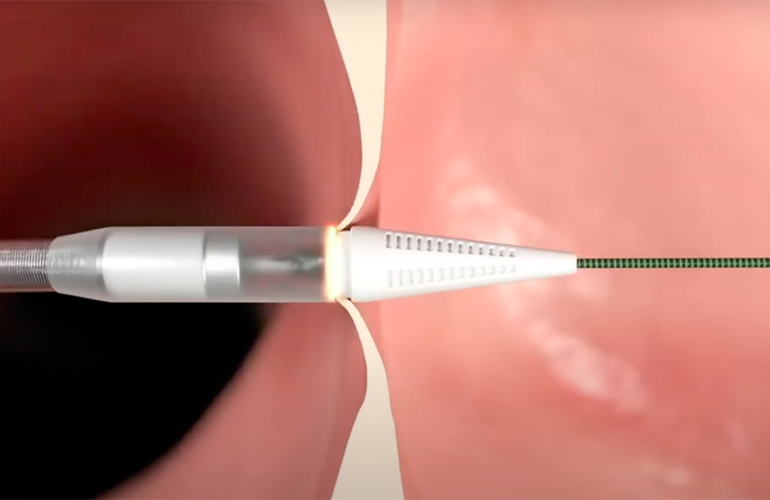
ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The same FDA advisory panel decided not to recommend Medtronic’s Symplicity Spyral RDN. Both devices are catheter-delivered and treat the nerves leading to the kidneys, but Medtronic’s device uses radiofrequency energy instead of ultrasound. In November, ReCor became the first company to win FDA approval for RDN, with Medtronic following about a week later.
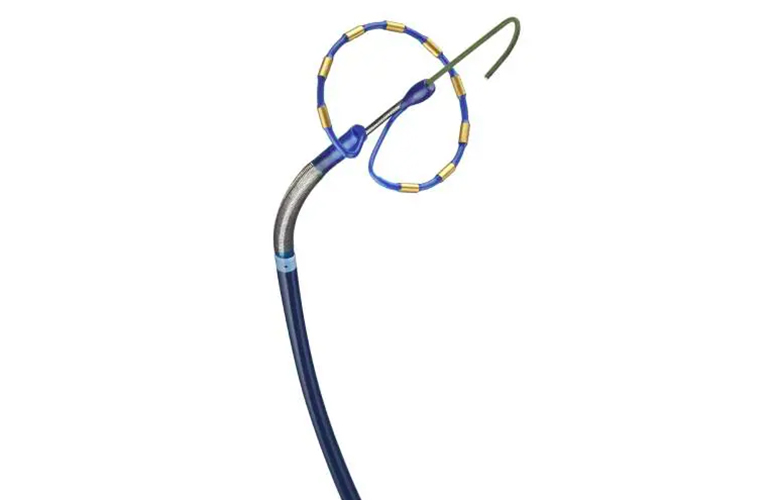
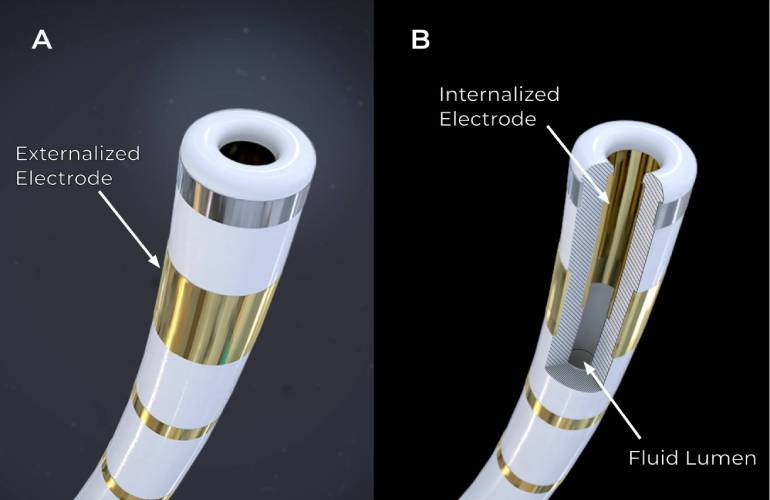
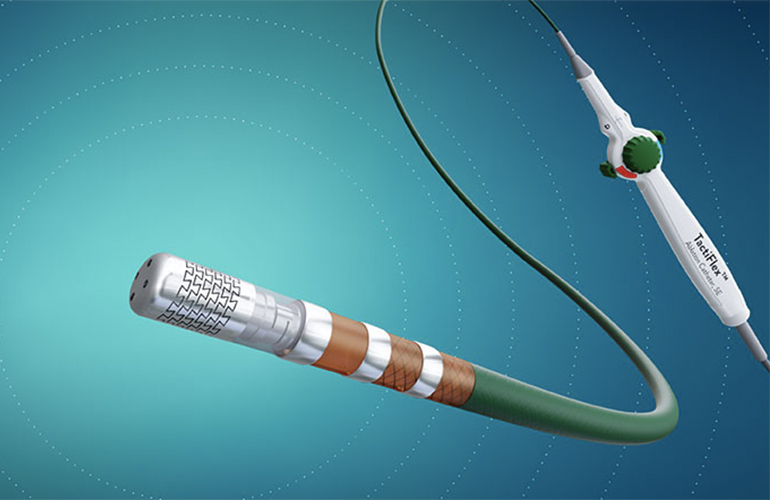
Medtronic was the first company to win FDA approval for pulsed field ablation (PFA) to treat atrial fibrillation in December. The path to that approval was much anticipated by Medical Tubing + Extrusion‘s readers this year – innovation news related to Medtronic’s PulseSelect PFA system comprised two of the top 10 most-read articles on MT+E.
Looking back at this year’s catheter coverage at Medical Tubing + Extrusion, we analyzed the most popular catheter innovations of 2023 based on reader interest. The top articles on MT+E were from a combination of major medtech manufacturers like Medtronic and J&J, and smaller private developers like Field Medical and Alleviant Medical. Catheter news that dominated our readers’ interest was largely cardiology-based, showing a growing interest in treating heart failure and atrial fibrillation. For comparison, our 2022 most-read catheter-based coverage covered a broader range of innovations like eustachian tube dilation balloons and stroke treatment.
Here are the top 10 catheter-based innovations that dominated the news on Medical Tubing + Extrusion in 2023.
(Click the + symbol to expand)