The FDA provided the Austin, Texas–based company with an Investigational Device Exemption for the clinical trial in November 2022. Researchers have designed the ALLAY-HF study to demonstrate the safety and effectiveness of the Alleviant System.
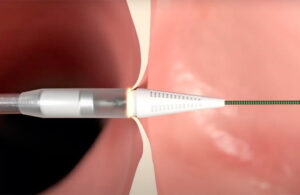
The Alleviant System is a transcatheter device that uses a short pulse of energy to create a durable passage between the heart’s left and right atrium. The goal is to reduce excess pressure within the left atrium. Alleviant Medical officials describe it as a less-invasive approach.
“Alleviant’s mission is to bring a safe, effective no-implant treatment option to millions of patients suffering from heart failure, and this highly informed study is a critical step forward,” CEO Adam Berman said in a news release. “We could not ask for a stronger group of institutional and strategic healthcare investors as we accelerate our global efforts to bring this technology to patients.”
Alleviant Medical study to enroll hundreds globally
ALLAY-HF will enroll 400 to 700 people around the world with chronic heart failure. Study enrollees will have a preserved (HFpEF) and mildly reduced (HFmrEF) ejection fraction (EF ≥ 40%) and remain symptomatic despite stable guideline-directed medical therapy. Researchers will conduct a composite primary endpoint analysis at one year.
Alleviant Medical says it already has studies involving 38 heart failure patients that confirmed shunt durability through six months and sustained improvement through 12 months.
S3 Ventures and RiverVest Venture Partners — along with investors Vensana Capital, Longview Ventures, TMC Venture Fund, and a strategic investor — are joined by Gilmartin Capital, ShangBay Capital and another undisclosed strategic investor in the new financing round.
“The Alleviant team is a real force in trailblazing novel solutions for heart failure,” said Brian R. Smith, managing director of S3 Ventures. “The team’s thoughtful approach to the ALLAY-HF trial demonstrates great insight into heart failure modalities and we remain bullish about their long-term prospects.”
