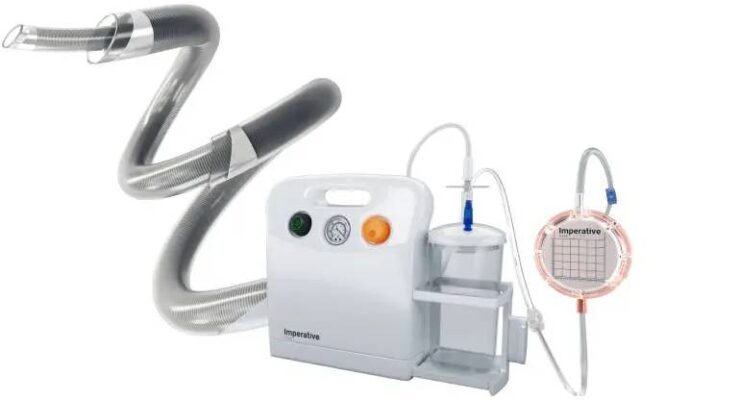
Imperative Care today announced study data evaluating aspiration with its Zoom system in stroke patients with M2 occlusions. Campbell, California–based Imperative Care designed Zoom for fast and effective clot removal from access through … [Read More...] about Imperative Care has positive stroke treatment study results
Main Content
Featured
Today on Medical Tubing & Extrusion
- Imperative Care has positive stroke treatment study results
- Medtronic enrolls first patient in study for Onyx liquid embolic system
- Medtronic to distribute Future Medical’s peripheral guidewires
- Real-world data backs Route 92 reperfusion system
- Johnson & Johnson MedTech gets updated FDA nod for Varipulse PFA
Medical Tubing & Extrusion
Imperative Care has positive stroke treatment study results
By Sean Whooley
Imperative Care today announced study data evaluating aspiration with its Zoom system in stroke patients with M2 occlusions. Campbell, California–based Imperative Care designed Zoom for fast and effective clot removal from access through … [Read More...] about Imperative Care has positive stroke treatment study results

Medtronic enrolls first patient in study for Onyx liquid embolic system
By Sean Whooley
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced the first patient enrolled in the PELE trial of its Onyx liquid embolic system (LES). PELE (Peripheral Onyx Liquid Embolic) evaluates the safety of Onyx LES for the … [Read More...] about Medtronic enrolls first patient in study for Onyx liquid embolic system

Medtronic to distribute Future Medical’s peripheral guidewires
By Sean Whooley
Medtronic (NYSE: MDT) today announced an exclusive U.S. distribution agreement for Future Medical Design peripheral guidewires. Japan-based Future offers specialty and workhorse guidewires. The agreement includes the first 400 cm, 0.018" peripheral … [Read More...] about Medtronic to distribute Future Medical’s peripheral guidewires
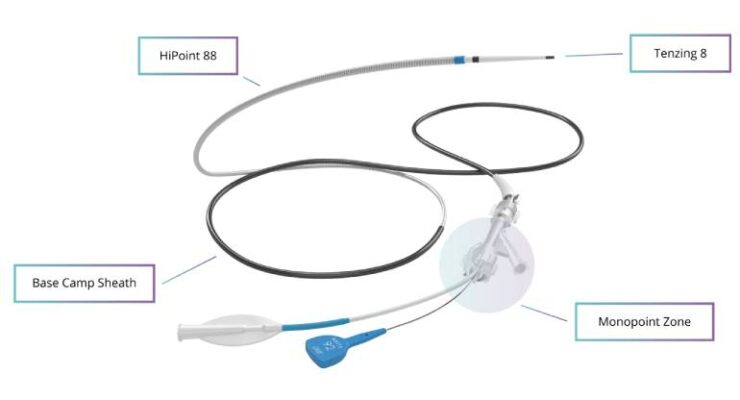
Real-world data backs Route 92 reperfusion system
By Sean Whooley
Route 92 Medical today announced results from a real-world study evaluating reperfusion outcomes with its HiPoint system. San Mateo, California-based Route 92’s HiPoint system includes the HiPoint 88 system powered by Tenzing 8, featuring the … [Read More...] about Real-world data backs Route 92 reperfusion system

Johnson & Johnson MedTech gets updated FDA nod for Varipulse PFA
By Sean Whooley
Johnson & Johnson MedTech today announced FDA approval for an update to its Varipulse platform's irrigation flow rate. The company said the update to its pulsed field ablation (PFA) platform reflects its commitment to the evolution of PFA … [Read More...] about Johnson & Johnson MedTech gets updated FDA nod for Varipulse PFA
NICE recommends Boston Scientific Farapulse pulsed field ablation tech
By Sean Whooley
Boston Scientific (NYSE:BSX) announced today that NICE issued guidance supporting the use of pulsed field ablation (PFA) for treating AFib. The National Institute for Health and Care Excellence (NICE), which provides evidence-based recommendations … [Read More...] about NICE recommends Boston Scientific Farapulse pulsed field ablation tech

Johnson & Johnson MedTech adds new CMO for its Electrophysiology business
By Sean Whooley
Johnson & Johnson MedTech (NYSE: JNJ) announced that it has a new chief medical & scientific officer for its Electrophysiology unit. Michael Bodner, group chair for Electrophysiology & Neurovascular, posted on LinkedIn that the company … [Read More...] about Johnson & Johnson MedTech adds new CMO for its Electrophysiology business